
Molecular orbital theory
Encyclopedia
In chemistry
, molecular orbital (MO) theory is a method for determining molecular structure in which electron
s are not assigned to individual bond
s between atom
s, but are treated as moving under the influence of the nuclei in the whole molecule. In this theory, each molecule has a set of molecular orbital
s, in which it is assumed that the molecular orbital wave function ψf may be written as a simple weighted sum of the n constituent atomic orbitals χi, according to the following equation:
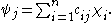
The cij coefficients may be determined numerically by substitution of this equation into the Schrödinger equation
and application of the variational principle
. This method is called the linear combination of atomic orbitals (LCAO ) approximation and is used in computational chemistry
. An additional unitary transformation can be applied on the system to accelerate the convergence in some computational schemes. Molecular orbital theory was seen as a competitor to valence bond theory
in the 1930s, before it was realized that the two methods are closely related and that when extended they become equivalent.
, Robert Mulliken, John C. Slater
, and John Lennard-Jones
. MO theory was originally called the Hund-Mulliken theory. The word orbital was introduced by Mulliken in 1932. By 1933, the molecular orbital theory had become accepted as a valid and useful theory. According to German physicist and physical chemist Erich Hückel
, the first quantitative use of molecular orbital theory was the 1929 paper of Lennard-Jones. The first accurate calculation of a molecular orbital wavefunction was that made by Charles Coulson
in 1938 on the hydrogen molecule. By 1950, molecular orbital
s were completely defined as eigenfunctions (wave functions) of the self-consistent field Hamiltonian
and it was at this point that molecular orbital theory became fully rigorous and consistent. This rigorous approach is known as the Hartree–Fock method for molecules although it had its origins in calculations on atoms. In calculations on molecules, the molecular orbitals are expanded in terms of an atomic orbital basis set
, leading to the Roothaan equations
. This led to the development of many ab initio quantum chemistry methods
. In parallel, molecular orbital theory was applied in a more approximate manner using some empirically derived parameters in methods now known as semi-empirical quantum chemistry methods
.
(MO) theory uses a linear combination of atomic orbitals
(LCAO) to represent molecular orbitals involving the whole molecule. These are often divided into bonding orbitals, anti-bonding
orbitals, and non-bonding orbital
s. A molecular orbital
is merely a Schrödinger orbital which includes several, but often only two nuclei. If this orbital is of type in which the electron(s) in the orbital have a higher probability of being between nuclei than elsewhere, the orbital will be a bonding orbital, and will tend to hold the nuclei together. If the electrons tend to be present in a molecular orbital in which they spend more time elsewhere than between the nuclei, the orbital will function as an anti-bonding orbital and will actually weaken the bond. Electrons in non-bonding orbitals tend to be in deep orbitals (nearly atomic orbital
s) associated almost entirely with one nucleus or the other, and thus they spend equal time between nuclei or not. These electrons neither contribute nor detract from bond strength.
Molecular orbitals are further divided according to the types of atomic orbitals combining to form a bond. These orbitals are results of electron-nucleus
interactions that are caused by the fundamental
force of electromagnetism
. Chemical substances will form a bond if their orbitals become lower in energy when they interact with each other. Different chemical bonds are distinguished that differ by electron cloud shape
and by energy level
s.
MO theory provides a global, delocalized perspective on chemical bonding. For example, in the MO theory for hypervalent molecules it is unnecessary to invoke a major role for d-orbitals, whereas valence bond theory
normally uses hybridization
with d-orbitals to explain hypervalency. In MO theory, any electron in a molecule may be found anywhere in the molecule, since quantum conditions allow electrons to travel under the influence of an arbitrarily large number of nuclei, so long as permitted by certain quantum rules. Although in MO theory some molecular orbitals may hold electrons which are more localized between specific pairs of molecular atoms, other orbitals may hold electrons which are spread more uniformly over the molecule. Thus, overall, bonding (and electrons) are far more delocalized (spread out) in MO theory, than is implied in valence bond (VB) theory. This makes MO theory more useful for the description of extended systems.
An example is that in the MO picture of benzene
, composed of a hexagonal ring of 6 carbon atoms. In this molecule, 24 of the 30 total valence bonding electrons are located in 12 σ (sigma) bonding orbitals which are mostly located between pairs of atoms (C-C or C-H), similar to the valence bond picture. However, in benzene the remaining 6 bonding electrons are located in 3 π (pi) molecular bonding orbitals that are delocalized around the ring. Two are in an MO which has equal contributions from all 6 atoms. The other two orbitals have vertical nodes at right angles to each other. As in the VB theory, all of these 6 delocalized pi electrons reside in a larger space which exists above and below the ring plane. All carbon-carbon bonds in benzene are chemically equivalent. In MO theory this is a direct consequence of the fact that the 3 molecular pi orbitals form a combination which evenly spreads the extra 6 electrons over 6 carbon atoms.
In molecules such as methane
, the 8 valence electrons are found in 4 MOs that are spread out over all 5 atoms. However, it is possible to approximate the MOs with 4 localized orbitals similar in shape to sp3 hybrid orbitals predicted by VB theory. This is often adequate for σ (sigma) bonds
, but it is not possible for the π (pi) orbitals
. However, the delocalized MO picture is more appropriate for ionization and spectroscopic predictions. Upon ionization of methane, a single electron is taken from the MO which surrounds the whole molecule, weakening all 4 bonds equally. VB theory would predict that one electron is removed for an sp3 orbital, resulting in the need for resonance between four valence bond structures, each of which has a one-electron bond.
As in benzene, in substances such as beta carotene, chlorophyll
or heme
, some electrons the π (pi) orbitals are spread out in molecular orbitals over long distances in a molecule, giving rise to light absorption in lower energies (visible colors), a fact which is observed. This and other spectroscopic data for molecules are better explained in MO theory, with an emphasis on electronic states associated with multicenter orbitals, including mixing of orbitals premised on principles of orbital symmetry matching. The same MO principles also more naturally explain some electrical phenomena, such as high electrical conductivity in the planar direction of the hexagonal atomic sheets that exist in graphite
. In MO theory, "resonance" (a mixing and blending of VB bond states) is a natural consequence of symmetry. For example, in graphite, as in benzene, it is not necessary to invoke the sp2 hybridization and resonance of VB theory, in order to explain electrical conduction. Instead, MO theory simply recognizes that some electrons in the graphite atomic sheets are completely delocalized
over arbitrary distances, and reside in very large molecular orbitals that cover an entire graphite sheet, and some electrons are thus as free to move and conduct electricity in the sheet plane, as if they resided in a metal
.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, molecular orbital (MO) theory is a method for determining molecular structure in which electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s are not assigned to individual bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s between atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s, but are treated as moving under the influence of the nuclei in the whole molecule. In this theory, each molecule has a set of molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s, in which it is assumed that the molecular orbital wave function ψf may be written as a simple weighted sum of the n constituent atomic orbitals χi, according to the following equation:
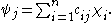
The cij coefficients may be determined numerically by substitution of this equation into the Schrödinger equation
Schrödinger equation
The Schrödinger equation was formulated in 1926 by Austrian physicist Erwin Schrödinger. Used in physics , it is an equation that describes how the quantum state of a physical system changes in time....
and application of the variational principle
Variational principle
A variational principle is a scientific principle used within the calculus of variations, which develops general methods for finding functions which minimize or maximize the value of quantities that depend upon those functions...
. This method is called the linear combination of atomic orbitals (LCAO ) approximation and is used in computational chemistry
Computational chemistry
Computational chemistry is a branch of chemistry that uses principles of computer science to assist in solving chemical problems. It uses the results of theoretical chemistry, incorporated into efficient computer programs, to calculate the structures and properties of molecules and solids...
. An additional unitary transformation can be applied on the system to accelerate the convergence in some computational schemes. Molecular orbital theory was seen as a competitor to valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
in the 1930s, before it was realized that the two methods are closely related and that when extended they become equivalent.
History
'Molecular orbital theory' was developed, in the years after valence bond theory had been established (1927), primarily through the efforts of Friedrich HundFriedrich Hund
Friedrich Hermann Hund was a German physicist from Karlsruhe known for his work on atoms and molecules.Hund worked at the Universities of Rostock, Leipzig, Jena, Frankfurt am Main, and Göttingen....
, Robert Mulliken, John C. Slater
John C. Slater
John Clarke Slater was a noted American physicist who made major contributions to the theory of the electronic structure of atoms, molecules and solids. This work is of ongoing importance in chemistry, as well as in many areas of physics. He also made major contributions to microwave electronics....
, and John Lennard-Jones
John Lennard-Jones
Sir John Edward Lennard-Jones KBE, FRS was a mathematician who was a professor of theoretical physics at Bristol University, and then of theoretical science at Cambridge University...
. MO theory was originally called the Hund-Mulliken theory. The word orbital was introduced by Mulliken in 1932. By 1933, the molecular orbital theory had become accepted as a valid and useful theory. According to German physicist and physical chemist Erich Hückel
Erich Hückel
Erich Armand Arthur Joseph Hückel was a German physicist and physical chemist. He is known for two major contributions:*The Debye–Hückel theory of electrolytic solutions...
, the first quantitative use of molecular orbital theory was the 1929 paper of Lennard-Jones. The first accurate calculation of a molecular orbital wavefunction was that made by Charles Coulson
Charles Coulson
Charles Alfred Coulson FRS was an applied mathematician, theoretical chemist and religious author.His major scientific work was as a pioneer of the application of the quantum theory of valency to problems of molecular structure, dynamics and reactivity...
in 1938 on the hydrogen molecule. By 1950, molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
s were completely defined as eigenfunctions (wave functions) of the self-consistent field Hamiltonian
Hamiltonian (quantum mechanics)
In quantum mechanics, the Hamiltonian H, also Ȟ or Ĥ, is the operator corresponding to the total energy of the system. Its spectrum is the set of possible outcomes when one measures the total energy of a system...
and it was at this point that molecular orbital theory became fully rigorous and consistent. This rigorous approach is known as the Hartree–Fock method for molecules although it had its origins in calculations on atoms. In calculations on molecules, the molecular orbitals are expanded in terms of an atomic orbital basis set
Basis set (chemistry)
A basis set in chemistry is a set of functions used to create the molecular orbitals, which are expanded as a linear combination of such functions with the weights or coefficients to be determined. Usually these functions are atomic orbitals, in that they are centered on atoms. Otherwise, the...
, leading to the Roothaan equations
Roothaan equations
The Roothaan equations are a representation of the Hartree-Fock equation in a non orthonormal basis set which can be of Gaussian-type or Slater-type. It applies to closed-shell molecules or atoms where all molecular orbitals or atomic orbitals, respectively, are doubly occupied. This is generally...
. This led to the development of many ab initio quantum chemistry methods
Ab initio quantum chemistry methods
Ab initio quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term ab initiowas first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excited states of benzene.The background is described by Parr...
. In parallel, molecular orbital theory was applied in a more approximate manner using some empirically derived parameters in methods now known as semi-empirical quantum chemistry methods
Semi-empirical quantum chemistry methods
Semi-empirical quantum chemistry methods are based on the Hartree-Fock formalism, but make many approximations and obtain some parameters from empirical data. They are very important in computational chemistry for treating large molecules where the full Hartree-Fock method without the...
.
Overview
Molecular orbitalMolecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
(MO) theory uses a linear combination of atomic orbitals
Linear combination of atomic orbitals molecular orbital method
A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunctions...
(LCAO) to represent molecular orbitals involving the whole molecule. These are often divided into bonding orbitals, anti-bonding
Antibonding
Antibonding is a type of chemical bonding. An antibonding orbital is a form of molecular orbital that is located outside the region of two distinct nuclei...
orbitals, and non-bonding orbital
Non-bonding orbital
A non-bonding orbital, also known as non-bonding molecular orbital and sometimes designated by the letter n in molecular orbital diagrams, is a molecular orbital whose occupation by electrons neither increases nor decreases the bond order between the involved atoms...
s. A molecular orbital
Molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
is merely a Schrödinger orbital which includes several, but often only two nuclei. If this orbital is of type in which the electron(s) in the orbital have a higher probability of being between nuclei than elsewhere, the orbital will be a bonding orbital, and will tend to hold the nuclei together. If the electrons tend to be present in a molecular orbital in which they spend more time elsewhere than between the nuclei, the orbital will function as an anti-bonding orbital and will actually weaken the bond. Electrons in non-bonding orbitals tend to be in deep orbitals (nearly atomic orbital
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
s) associated almost entirely with one nucleus or the other, and thus they spend equal time between nuclei or not. These electrons neither contribute nor detract from bond strength.
Molecular orbitals are further divided according to the types of atomic orbitals combining to form a bond. These orbitals are results of electron-nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
interactions that are caused by the fundamental
Fundamental interaction
In particle physics, fundamental interactions are the ways that elementary particles interact with one another...
force of electromagnetism
Electromagnetism
Electromagnetism is one of the four fundamental interactions in nature. The other three are the strong interaction, the weak interaction and gravitation...
. Chemical substances will form a bond if their orbitals become lower in energy when they interact with each other. Different chemical bonds are distinguished that differ by electron cloud shape
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
and by energy level
Energy level
A quantum mechanical system or particle that is bound -- that is, confined spatially—can only take on certain discrete values of energy. This contrasts with classical particles, which can have any energy. These discrete values are called energy levels...
s.
MO theory provides a global, delocalized perspective on chemical bonding. For example, in the MO theory for hypervalent molecules it is unnecessary to invoke a major role for d-orbitals, whereas valence bond theory
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
normally uses hybridization
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
with d-orbitals to explain hypervalency. In MO theory, any electron in a molecule may be found anywhere in the molecule, since quantum conditions allow electrons to travel under the influence of an arbitrarily large number of nuclei, so long as permitted by certain quantum rules. Although in MO theory some molecular orbitals may hold electrons which are more localized between specific pairs of molecular atoms, other orbitals may hold electrons which are spread more uniformly over the molecule. Thus, overall, bonding (and electrons) are far more delocalized (spread out) in MO theory, than is implied in valence bond (VB) theory. This makes MO theory more useful for the description of extended systems.
An example is that in the MO picture of benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, composed of a hexagonal ring of 6 carbon atoms. In this molecule, 24 of the 30 total valence bonding electrons are located in 12 σ (sigma) bonding orbitals which are mostly located between pairs of atoms (C-C or C-H), similar to the valence bond picture. However, in benzene the remaining 6 bonding electrons are located in 3 π (pi) molecular bonding orbitals that are delocalized around the ring. Two are in an MO which has equal contributions from all 6 atoms. The other two orbitals have vertical nodes at right angles to each other. As in the VB theory, all of these 6 delocalized pi electrons reside in a larger space which exists above and below the ring plane. All carbon-carbon bonds in benzene are chemically equivalent. In MO theory this is a direct consequence of the fact that the 3 molecular pi orbitals form a combination which evenly spreads the extra 6 electrons over 6 carbon atoms.
In molecules such as methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, the 8 valence electrons are found in 4 MOs that are spread out over all 5 atoms. However, it is possible to approximate the MOs with 4 localized orbitals similar in shape to sp3 hybrid orbitals predicted by VB theory. This is often adequate for σ (sigma) bonds
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
, but it is not possible for the π (pi) orbitals
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
. However, the delocalized MO picture is more appropriate for ionization and spectroscopic predictions. Upon ionization of methane, a single electron is taken from the MO which surrounds the whole molecule, weakening all 4 bonds equally. VB theory would predict that one electron is removed for an sp3 orbital, resulting in the need for resonance between four valence bond structures, each of which has a one-electron bond.
As in benzene, in substances such as beta carotene, chlorophyll
Chlorophyll
Chlorophyll is a green pigment found in almost all plants, algae, and cyanobacteria. Its name is derived from the Greek words χλωρος, chloros and φύλλον, phyllon . Chlorophyll is an extremely important biomolecule, critical in photosynthesis, which allows plants to obtain energy from light...
or heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
, some electrons the π (pi) orbitals are spread out in molecular orbitals over long distances in a molecule, giving rise to light absorption in lower energies (visible colors), a fact which is observed. This and other spectroscopic data for molecules are better explained in MO theory, with an emphasis on electronic states associated with multicenter orbitals, including mixing of orbitals premised on principles of orbital symmetry matching. The same MO principles also more naturally explain some electrical phenomena, such as high electrical conductivity in the planar direction of the hexagonal atomic sheets that exist in graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
. In MO theory, "resonance" (a mixing and blending of VB bond states) is a natural consequence of symmetry. For example, in graphite, as in benzene, it is not necessary to invoke the sp2 hybridization and resonance of VB theory, in order to explain electrical conduction. Instead, MO theory simply recognizes that some electrons in the graphite atomic sheets are completely delocalized
Delocalized electron
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or one covalent bond....
over arbitrary distances, and reside in very large molecular orbitals that cover an entire graphite sheet, and some electrons are thus as free to move and conduct electricity in the sheet plane, as if they resided in a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
.
See also
- Ab initio quantum chemistry methodsAb initio quantum chemistry methodsAb initio quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term ab initiowas first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excited states of benzene.The background is described by Parr...
- Atomic orbitalAtomic orbitalAn atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus...
- Configuration interactionConfiguration interactionConfiguration interaction is a post-Hartree–Fock linear variational method for solving the nonrelativistic Schrödinger equation within the Born–Oppenheimer approximation for a quantum chemical multi-electron system. Mathematically, configuration simply describes the linear combination...
- Coupled clusterCoupled clusterCoupled cluster is a numerical technique used for describing many-body systems. Its most common use is as one of several quantum chemical post-Hartree–Fock ab initio quantum chemistry methods in the field of computational chemistry...
- Hartree–Fock
- Molecular orbitalMolecular orbitalIn chemistry, a molecular orbital is a mathematical function describing the wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The term "orbital" was first...
- MO diagramMO diagramA molecular orbital diagram, or MO diagram for short, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method in particular...
- Møller–Plesset perturbation theory
- Quantum chemistry computer programsQuantum chemistry computer programsQuantum chemistry computer programs are used in computational chemistry to implement the methods of quantum chemistry. Most include the Hartree–Fock and some post-Hartree–Fock methods. They may also include density functional theory , molecular mechanics or semi-empirical quantum...
- Semi-empirical quantum chemistry methodsSemi-empirical quantum chemistry methodsSemi-empirical quantum chemistry methods are based on the Hartree-Fock formalism, but make many approximations and obtain some parameters from empirical data. They are very important in computational chemistry for treating large molecules where the full Hartree-Fock method without the...
External links
- Molecular Orbital Theory - Purdue University
- Molecular Orbital Theory - Sparknotes
- Molecular Orbital Theory - Mark Bishop's Chemistry Site
- Introduction to MO Theory - Queen Mary, London University
- Molecular Orbital Theory - a related terms table

