Nitro compound
Encyclopedia

Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s that contain one or more nitro functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s (-2). They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophore
Explosophore
Explosophores are functional groups in organic chemistry that give organic compounds explosive properties. The term was first coined by Russian chemist V. Pletz in 1935. and originally mistranslated in some articles as "plosophore"...
s (functional group that makes a compound explosive) used globally. This property of both nitro and nitrate groups is because their thermal decomposition yields molecular nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
N2 gas plus considerable energy, due to the high strength of the bond in molecular nitrogen.
Aromatic nitro compounds are typically synthesized by the action of a mixture of nitric
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
s on an organic molecule. The one produced on the largest scale, by far, is nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
. Many explosives are produced by nitration including trinitrophenol (picric acid), trinitrotoluene (TNT), and trinitroresorcinol (styphnic acid).
Occurrence in nature
ChloramphenicolChloramphenicol
Chloramphenicol is a bacteriostatic antimicrobial that became available in 1949. It is considered a prototypical broad-spectrum antibiotic, alongside the tetracyclines, and as it is both cheap and easy to manufacture it is frequently found as a drug of choice in the third world.Chloramphenicol is...
is a rare example of a naturally occurring
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
nitro compound. At least some naturally occurring nitro groups arise by the oxidation of amino groups. 2-Nitrophenol is an aggregation pheromone
Pheromone
A pheromone is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting outside the body of the secreting individual to impact the behavior of the receiving individual...
of ticks.
Only two examples of aliphatic nitro compounds are known in nature. 3-Nitropropionic acid found in fungi
Fungus
A fungus is a member of a large group of eukaryotic organisms that includes microorganisms such as yeasts and molds , as well as the more familiar mushrooms. These organisms are classified as a kingdom, Fungi, which is separate from plants, animals, and bacteria...
and plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s (Indigofera
Indigofera
Indigofera is a large genus of about 700 species of flowering plants belonging to the family Fabaceae.The species are mostly shrubs, though some are herbaceous, and a few can become small trees up to tall. Most are dry-season or winter deciduous. The leaves are pinnate with 5–31 leaflets and the...
). Nitropentadecene
Nitropentadecene
Nitropentadecene, or more precisely -1-nitropentadec-1-ene, is a highly toxic unsaturated nitroalkene, the only aliphatic nitro compound known to be synthesized by insects. It is produced by termite soldiers of Prorhinotermes genus as a defensive chemical...
is a defense compound found in termite
Termite
Termites are a group of eusocial insects that, until recently, were classified at the taxonomic rank of order Isoptera , but are now accepted as the epifamily Termitoidae, of the cockroach order Blattodea...
s.
Many flavin-dependent enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s are capable of oxidizing aliphatic nitro compounds to less-toxic aldehydes and ketones. Nitroalkane oxidase
Nitroalkane oxidase
In enzymology, a nitroalkane oxidase is an enzyme that catalyzes the chemical reactionThe 3 substrates of this enzyme are nitroalkane, H2O, and O2, whereas its 4 products are aldehyde, ketone, nitrite, and H2O2....
and 3-nitropropionate oxidase oxidize aliphatic nitro compounds exclusively, whereas other enzymes such as glucose oxidase
Glucose oxidase
The glucose oxidase enzyme is an oxido-reductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. In cells, it aids in breaking the sugar down into its metabolites....
have other physiological substrates.
Aliphatic nitro compounds
NitromethaneNitromethane
Nitromethane is an organic compound with the chemical formula . It is the simplest organic nitro compound. It is a slightly viscous, highly polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent...
, nitroethane
Nitroethane
Nitroethane is an organic compound having the chemical formula C2H5NO2. Similar in many regards to nitromethane, nitroethane is an oily liquid at standard temperature and pressure. Pure nitroethane is colourless and has a fruity odor.- Preparation :...
, and nitropropanes are produced industrially by treating propane with nitric acid in the gas phase. Nitromethane can be produced in the laboratory by treating sodium chloroacetate
Chloroacetic acid
Chloroacetic acid, industrially known as monochloroacetic acid is the organochlorine compound with the formula ClCH2CO2H. This carboxylic acid is a useful building-block in organic synthesis.-Production:...
with sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
, forming sodium bicarbonate
Sodium bicarbonate
Sodium bicarbonate or sodium hydrogen carbonate is the chemical compound with the formula Na HCO3. Sodium bicarbonate is a white solid that is crystalline but often appears as a fine powder. It has a slightly salty, alkaline taste resembling that of washing soda . The natural mineral form is...
and sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
as byproducts.
Aromatic nitro compounds
In a classic electrophilic substitutionElectrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a group in a compound, typically but not always hydrogen. Electrophilic aromatic substitution is characteristic of aromatic compounds and is an important way of introducing functional groups onto benzene...
reaction, nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
produce the nitronium ion, which reacts with aromatic compounds in aromatic nitration. Another method, starting from halogenated phenols, is the Zinke nitration
Zinke nitration
The Zincke nitration is an organic reaction in which a bromine substituent of a phenol or cresol is replaced by a nitro group by treatment with nitrous acid or sodium nitrite. The reaction is a manifestation of nucleophilic aromatic substitution...
.
Reactions
Nitro compounds participate in several organic reactionOrganic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s, the most important being their reduction to the corresponding amines:
- RNO2 + 3 H2 → RNH2 + 2 H2O
Virtually all aromatic amines (aniline
Aniline
Aniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
s) are derived from nitroaromatics.
Aliphatic nitro compounds
- Aliphatic nitro compounds are reduced to amineAmineAmines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s with hydrochloric acidHydrochloric acidHydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
and an ironIronIron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
catalyst - NitronateNitronateA nitronate in organic chemistry is a functional group with the general structure R1R2C=N+2-).It is the anion of a nitronic acid, a tautomeric form of a nitro compound. A nitronic acid is also called a aci form...
s are a tautomeric form of aliphatic nitro compounds. - HydrolysisHydrolysisHydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the salts of nitro compounds yield aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s or ketoneKetoneIn organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s in the Nef reactionNef reactionThe Nef reaction is an organic reaction describing the acid hydrolysis of a salt of a primary or secondary nitroalkane to an aldehyde or a ketone and nitrous oxide .... - Nitromethane adds to aldehydeAldehydeAn aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s in 1,2-addition in the nitroaldol reactionNitroaldol reactionThe Henry Reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by L. Henry, it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-Nitro alcohols... - Nitromethane adds to alpha-beta unsaturated carbonyl compounds as a 1,4-addition in the Michael reactionMichael reactionThe Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
as a Michael donor - Nitroethylene is a Michael acceptor in a Michael reactionMichael reactionThe Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an alpha, beta unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds...
with enolate compounds - In nucleophilic aliphatic substitutionNucleophilic substitutionIn organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
sodium nitriteSodium nitriteSodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
(NaNO2) replaces an alkyl halide. In the so-called ter Meer reaction (1876) named after Edmund ter MeerEdmund ter MeerEdmund ter Meer was a German chemist who discovered the ter Meer reaction and founded in 1877 the ter Meer dye company in Uerdingen. After the fusion with the aniline factory of Julius Weiler the Weiler-ter Meer company was formed. This company later became part of the Bayer company...
. The reactant is a 1,1-halonitroalkane:
- In one study, a reaction mechanismReaction mechanismIn chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is proposed in which in the first slow step a protonHydroniumIn chemistry, a hydronium ion is the cation , a type of oxonium ion produced by protonation of water. This cation is often used to represent the nature of the proton in aqueous solution, where the proton is highly solvated...
is abstracted from nitroalkane 1 to a carbanionCarbanionA carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
2 followed by protonationProtonationIn chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
to a nitronateNitronateA nitronate in organic chemistry is a functional group with the general structure R1R2C=N+2-).It is the anion of a nitronic acid, a tautomeric form of a nitro compound. A nitronic acid is also called a aci form...
3 and finally nucleophilic displacement of chlorine based on an experimentally observed hydrogen kinetic isotope effectKinetic isotope effectThe kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
of 3.3. When the same reactant is reacted with potassium hydroxidePotassium hydroxidePotassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
the reaction product is the 1,2-dinitro dimer
Aromatic nitro compounds
- Reduction of aromatic nitro compounds with hydrogenHydrogenHydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
gas over a platinumPlatinumPlatinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
catalyst gives anilineAnilineAniline, phenylamine or aminobenzene is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the prototypical aromatic amine. Being a precursor to many industrial chemicals, its main use is in the manufacture of precursors to polyurethane...
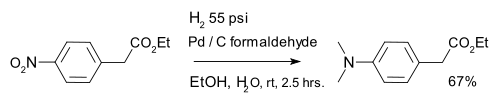
s. A variation is formation of a dimethylaminoarene with palladium on carbonPalladium on carbonPalladium on carbon, often referred to as Pd/C, is a form of palladium used for catalysis. It is usually used for catalytic hydrogenations in organic chemistry...
and formaldehydeFormaldehydeFormaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
:
- The Leimgruber-BatchoLeimgruber-Batcho indole synthesisThe Leimgruber–Batcho indole synthesis is a series of organic reactions that produce indoles from o-nitrotoluenes 1. The first step is the formation of an enamine 2 using N,N-dimethylformamide dimethyl acetal and pyrrolidine...
, BartoliBartoli indole synthesisThe Bartoli indole synthesis is the chemical reaction of ortho-substituted nitroarenes with vinyl grignard reagents to form substituted indoles....
and Baeyer-EmmerlingBaeyer-Emmerling indole synthesisThe Baeyer-Emmerling indole synthesis is a method for synthesizing indole from a ortho-nitrocinnamic acid and iron powder in strongly basic solution. This reaction was discovered by Adolf von Baeyer and A. Emmerling in 1869....
indole syntheses begin with aromatic nitro compounds.
- IndigoIndigo dyeIndigo dye is an organic compound with a distinctive blue color . Historically, indigo was a natural dye extracted from plants, and this process was important economically because blue dyes were once rare. Nearly all indigo dye produced today — several thousand tons each year — is synthetic...
can be synthesized in a condensation reaction from ortho-nitrobenzaldehyde and acetoneAcetoneAcetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
in strongly basic conditions in a reaction known as the Baeyer-Drewson indigo synthesisBaeyer-Drewson indigo synthesisThe Baeyer–Drewson indigo synthesis is an organic reaction in which indigo is prepared from 2-nitrobenzaldehyde and acetone The reaction is classified as a Aldol condensation...
- The presence of nitro groups retards electrophilic aromatic substitutionElectrophilic aromatic substitutionElectrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile...
but facilitates nucleophilic aromatic substitutionNucleophilic aromatic substitutionright|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
because they are highly electron-withdrawing.
See also
- Functional groupFunctional groupIn organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
- Reduction of nitro compoundsReduction of nitro compoundsThe chemical reactions described as reduction of nitro compounds can be facilitated by many different reagents and reaction conditions. Historically, the nitro group was one of the first functional groups to be reduced, due to the ease of nitro-group reduction....
- NitrationNitrationNitration is a general chemical process for the introduction of a nitro group into a chemical compound. The dominant application of nitration is for the production of nitrobenzene, the precursor to methylene diphenyl diisocyanate...
- NitriteNitriteThe nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
Also an NO2 group, but bonds differently.