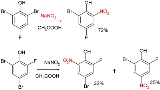
Zinke nitration
Encyclopedia
The Zincke nitration is an organic reaction
in which a bromine
substituent
of a phenol
or cresol
is replaced by a nitro
group by treatment with nitrous acid
or sodium nitrite
. The reaction is a manifestation of nucleophilic aromatic substitution
. The reaction is named after Theodor Zincke
.
Two examples:
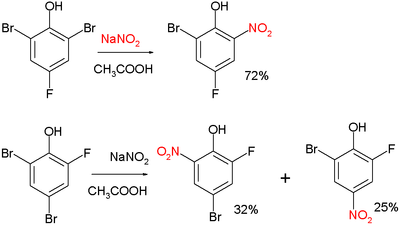 and:
and:
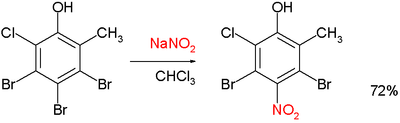 The Zincke nitration should not be confused with the Zincke-Suhl reaction
The Zincke nitration should not be confused with the Zincke-Suhl reaction
or the Zincke reaction
.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which a bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
of a phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
or cresol
Cresol
Cresols are organic compounds which are methylphenols. They are a widely occurring natural and manufactured group of aromatic organic compounds which are categorized as phenols . Depending on the temperature, cresols can be solid or liquid because they have melting points not far from room...
is replaced by a nitro
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
group by treatment with nitrous acid
Nitrous acid
Nitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water...
or sodium nitrite
Sodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
. The reaction is a manifestation of nucleophilic aromatic substitution
Nucleophilic aromatic substitution
right|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
. The reaction is named after Theodor Zincke
Theodor Zincke
Theodor Zincke was a German chemist and the academic adviser of Otto Hahn.-Life:Theodor Zincke was born in Uelzen on 19 May 1843. He became a pharmacist and graduated in Göttingen with his Staatsexamen. He began studying chemistry with Friedrich Wöhler and received his Ph.D in 1869...
.
Two examples:


Zincke-Suhl reaction
The Zincke-Suhl reaction is a special case of a Friedel-Crafts alkylation and was first described by Theodor Zincke and Suhl.The classic example of this reaction is the conversion of p-cresol to a cyclohexadienone . Melvin Newman, a scientist from the U.S...
or the Zincke reaction
Zincke reaction
The Zincke reaction is an organic reaction in which a pyridine is transformed into a pyridinium salt by reaction with 2,4-dinitro-chlorobenzene and a primary amine, named after Theodor Zincke....
.

