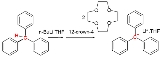
Carbanion
Overview
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
has an unshared pair of electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s and bears a negative charge usually with three substituents for a total of eight valence electron
Valence electron
In chemistry, valence electrons are the electrons of an atom that can participate in the formation of chemical bonds with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single...
s. The carbanion exists in a trigonal pyramidal
Trigonal pyramid (chemistry)
In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base. When all three atoms at the corners are identical, the molecule belongs to point group C3v. One example of a molecule with a trigonal pyramidal geometry is ammonia...
geometry. Formally a carbanion is the conjugate base of a carbon acid.
- R3C-H + B− → R3C− + H-B
where B stands for the base.
Unanswered Questions

