
Cathode
Encyclopedia
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
through which electric current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
flows out of a polarized electrical device. Mnemonic
Mnemonic
A mnemonic , or mnemonic device, is any learning technique that aids memory. To improve long term memory, mnemonic systems are used to make memorization easier. Commonly encountered mnemonics are often verbal, such as a very short poem or a special word used to help a person remember something,...
: CCD (Cathode Current Departs).
Cathode polarity
Electrical polarity
Electrical polarity is present in every electrical circuit. Electrons flow from the negative pole to the positive pole. In a direct current circuit, one pole is always negative, the other pole is always positive and the electrons flow in one direction only...
is not always negative. Although positively charged cations
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
always move towards the cathode (hence their name) and/or negatively charged anions
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
move away from it, cathode polarity depends on the device type, and can even vary according to the operating mode. In a device which consumes power, the cathode is negative, and in a device which provides power, the cathode is positive:
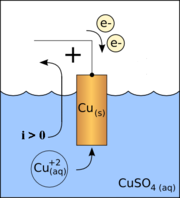
- In a discharging battery or a galvanic cellGalvanic cellA Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
the cathode is the positive terminal since that is where the current flows out of the device (see drawing). This outward current is carried internally by positive ions moving from the electrolyteElectrolyteIn chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
to the positive cathode (chemical energy is responsible for this "uphill" motion). It is continued externally by electrons moving inwards, negative charge moving one way constituting positive current flowing the other way. For example, the Daniell galvanic cellDaniell cellThe Daniell cell was invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode...
's copper electrode is the positive terminal and the cathode. - In a recharging battery, or an electrolytic cellElectrolytic cellAn electrolytic cell decomposes chemical compounds by means of electrical energy, in a process called electrolysis; the Greek word lysis means to break up. The result is that the chemical energy is increased...
, the cathode is the negative terminal, which sends current back to the external generator. For example, reversing the current direction in a Daniell galvanic cellDaniell cellThe Daniell cell was invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consisted of a copper pot filled with a copper sulfate solution, in which was immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode...
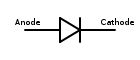
would produce an electrolytic cell, where the copper electrode is the positive terminal and the anode. - In a diodeDiodeIn electronics, a diode is a type of two-terminal electronic component with a nonlinear current–voltage characteristic. A semiconductor diode, the most common type today, is a crystalline piece of semiconductor material connected to two electrical terminals...
, it is the negative terminal at the pointed end of the arrow symbol, where current flows out of the device. Note: electrode naming for diodes is always based on the direction of the forward current (that of the arrow, in which the current flows "most easily"), even for types such as Zener diodeZener diodeA Zener diode is a special kind of diode which allows current to flow in the forward direction in the same manner as an ideal diode, but will also permit it to flow in the reverse direction when the voltage is above a certain value known as the breakdown voltage, "Zener knee voltage" or "Zener...
s or solar cellSolar cellA solar cell is a solid state electrical device that converts the energy of light directly into electricity by the photovoltaic effect....
s where the current of interest is the reverse current. - In vacuum tubeVacuum tubeIn electronics, a vacuum tube, electron tube , or thermionic valve , reduced to simply "tube" or "valve" in everyday parlance, is a device that relies on the flow of electric current through a vacuum...
s (including cathode ray tubeCathode ray tubeThe cathode ray tube is a vacuum tube containing an electron gun and a fluorescent screen used to view images. It has a means to accelerate and deflect the electron beam onto the fluorescent screen to create the images. The image may represent electrical waveforms , pictures , radar targets and...
s) it is the negative terminal where electrons flow in from the wiring and through the tube's near vacuum, constituting a positive current flowing out of the device.
An electrode through which current flows the other way (into the device) is termed an anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
.
Etymology
The word was coined in 1834 from the GreekGreek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
κάθοδος (kathodos), 'descent' or 'way down', by William Whewell
William Whewell
William Whewell was an English polymath, scientist, Anglican priest, philosopher, theologian, and historian of science. He was Master of Trinity College, Cambridge.-Life and career:Whewell was born in Lancaster...
, who had been consulted by Michael Faraday
Michael Faraday
Michael Faraday, FRS was an English chemist and physicist who contributed to the fields of electromagnetism and electrochemistry....
over some new names needed to complete a paper on the recently discovered process of electrolysis. In that paper Faraday explained that when an electrolytic cell is oriented so that electric current traverses the "decomposing body" (electrolyte) in a direction "from East to West, or, which will strengthen this help to the memory, that in which the sun appears to move", the cathode is where the current leaves the electrolyte, on the West side: "kata downwards, `odos a way ; the way which the sun sets".
The use of 'West' to mean the 'out' direction (actually 'out' → 'West' → 'sunset' → 'down', i.e. 'out of view') may appear unnecessarily contrived. Previously, as related in the first reference cited above, Faraday had used the more straightforward term "exode" (the doorway where the current exits). His motivation for changing it to something meaning 'the West electrode' (other candidates had been "westode", "occiode" and "dysiode") was to make it immune to a possible later change in the direction convention for current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
, whose exact nature was not known at the time. The reference he used to this effect was the Earth's magnetic field direction, which at that time was believed to be invariant. He fundamentally defined his arbitrary orientation for the cell as being that in which the internal current would run parallel to and in the same direction as a hypothetical magnetizing current loop
Solenoid
A solenoid is a coil wound into a tightly packed helix. In physics, the term solenoid refers to a long, thin loop of wire, often wrapped around a metallic core, which produces a magnetic field when an electric current is passed through it. Solenoids are important because they can create...
around the local line of latitude which would induce a magnetic dipole
Dipole
In physics, there are several kinds of dipoles:*An electric dipole is a separation of positive and negative charges. The simplest example of this is a pair of electric charges of equal magnitude but opposite sign, separated by some distance. A permanent electric dipole is called an electret.*A...
field oriented like the Earth's. This made the internal current East to West as previously mentioned, but in the event of a later convention change it would have become West to East, so that the West electrode would not have been the 'way out' any more. Therefore "exode" would have become inappropriate, whereas "cathode" meaning 'West electrode' would have remained correct with respect to the unchanged direction of the actual phenomenon underlying the current, then unknown but, he thought, unambiguously defined by the magnetic reference. In retrospect the name change was unfortunate, not only because the Greek roots alone do not reveal the cathode's function any more, but more importantly because, as we now know, the Earth's magnetic field direction on which the "cathode" term is based is subject to reversals
Geomagnetic reversal
A geomagnetic reversal is a change in the Earth's magnetic field such that the positions of magnetic north and magnetic south are interchanged. The Earth's field has alternated between periods of normal polarity, in which the direction of the field was the same as the present direction, and reverse...
whereas the current
Electric current
Electric current is a flow of electric charge through a medium.This charge is typically carried by moving electrons in a conductor such as wire...
direction convention on which the "exode" term was based has no reason to change in the future.
Since the later discovery of the electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
, an easier to remember, and more durably technically correct (although historically false), etymology has been suggested: cathode, from the Greek kathodos, 'way down', 'the way (down) into the cell (or other device) for electrons'.
Flow of electrons
The flow of electrons is always from anode to cathode outside of the cell or device, regardless of the cell or device type and operating mode, with the exception of diodeDiode
In electronics, a diode is a type of two-terminal electronic component with a nonlinear current–voltage characteristic. A semiconductor diode, the most common type today, is a crystalline piece of semiconductor material connected to two electrical terminals...
s where electrode naming always assumes current flows in the forward direction (that of the arrow symbol), i.e., electrons flow in the opposite direction, even when the diode reverse-conducts either by accident (breakdown of a normal diode) or by design (breakdown of a Zener diode, photo-current of a photodiode or solar cell).
Chemistry cathode
In chemistryChemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, a cathode is the electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
of an electrochemical cell
Electrochemical cell
An electrochemical cell is a device capable of either deriving electrical energy from chemical reactions, or facilitating chemical reactions through the introduction of electrical energy. A common example of an electrochemical cell is a standard 1.5-volt "battery"...
at which reduction occurs; a useful mnemonic
Mnemonic
A mnemonic , or mnemonic device, is any learning technique that aids memory. To improve long term memory, mnemonic systems are used to make memorization easier. Commonly encountered mnemonics are often verbal, such as a very short poem or a special word used to help a person remember something,...
to remember this is AnOx RedCat. The cathode can be negative as when the cell is electrolytic (where electrical energy provided to the cell is being used to decompose chemical compounds); or positive as when the cell is galvanic (where chemical reactions are used to generate electrical energy). The cathode supplies electrons to the positively charged cations which flow to it from the electrolyte (even if the cell is galvanic, i.e., when the cathode is positive and therefore would be expected to repel the positively charged cations; this is due to electrode potential
Electrode potential
Electrode potential, E, in electrochemistry, according to an IUPAC definition, is the electromotive force of a cell built of two electrodes:* on the left-hand side is the standard hydrogen electrode, and...
relative to the electrolyte solution being different for the anode and cathode metal/electrolyte systems in a galvanic cell
Galvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
).
The cathodic current, in electrochemistry
Electrochemistry
Electrochemistry is a branch of chemistry that studies chemical reactions which take place in a solution at the interface of an electron conductor and an ionic conductor , and which involve electron transfer between the electrode and the electrolyte or species in solution.If a chemical reaction is...
, is the flow of electrons from the cathode interface to a species in solution. The anodic current is the flow of electrons into the anode from a species in solution.
Electrolytic cell
In an electrolytic cellElectrolytic cell
An electrolytic cell decomposes chemical compounds by means of electrical energy, in a process called electrolysis; the Greek word lysis means to break up. The result is that the chemical energy is increased...
, the cathode is where the negative polarity is applied to drive the cell. Common results of reduction at the cathode are hydrogen gas or pure metal from metal ions. When discussing the relative reducing power of two redox agents, the couple for generating the more reducing species is said to be more "cathodic" with respect to the more easily reduced reagent.
Galvanic cell
In a galvanic cellGalvanic cell
A Galvanic cell, or Voltaic cell, named after Luigi Galvani, or Alessandro Volta respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reaction taking place within the cell...
, the cathode is where the positive pole is connected to allow the circuit to be completed: as the anode of the galvanic cell gives off electrons, they return from the circuit into the cell through the cathode.
Electroplating metal cathode
When metal ions are reduced from ionic solution, they form a pure metal surface on the cathode. Items to be plated with pure metal are attached to and become part of the cathode in the electrolytic solution.Electronics and physics cathode
In physicsPhysics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
or electronics
Electronics
Electronics is the branch of science, engineering and technology that deals with electrical circuits involving active electrical components such as vacuum tubes, transistors, diodes and integrated circuits, and associated passive interconnection technologies...
, a cathode is an electrode that emits electrons into the device.
Vacuum tubes
In a vacuum tube or electronic vacuum system, the cathode emits free electrons. Electrons are extracted from metal electrodes either by heating the electrode, causing thermionic emissionThermionic emission
Thermionic emission is the heat-induced flow of charge carriers from a surface or over a potential-energy barrier. This occurs because the thermal energy given to the carrier overcomes the binding potential, also known as work function of the metal. The charge carriers can be electrons or ions, and...
, or by applying a strong electric field and causing field electron emission. Electrons can also be emitted from the electrode
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
s of certain metals when light of frequency
Frequency
Frequency is the number of occurrences of a repeating event per unit time. It is also referred to as temporal frequency.The period is the duration of one cycle in a repeating event, so the period is the reciprocal of the frequency...
greater than the threshold frequency falls on it. This effect is called photoelectric emission.
Cold cathodes and hot cathodes
Cathodes used for field electron emission in vacuum tubes are called cold cathodeCold cathode
A cold cathode is a cathode used within nixie tubes, gas discharge lamps, discharge tubes, and some types of vacuum tube which is not electrically heated by the circuit to which it is connected...
s. Heated electrodes or hot cathode
Hot cathode
In vacuum tubes, a hot cathode is a cathode electrode which emits electrons due to thermionic emission. In the accelerator community, these are referred to as thermionic cathodes. The heating element is usually an electrical filament...
s, frequently called filaments, are much more common. Most radios and television sets prior to the 1970s used filament-heated-cathode electron tubes for signal selection and processing; to this day, a hot cathode forms the source of the electron beam(s) in cathode ray tubes in many television sets and computer monitors. Hot electron emitters are also used as the electrodes in fluorescent lamps and in the source tubes of X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
machines.
Diodes
Semiconductor device
Semiconductor devices are electronic components that exploit the electronic properties of semiconductor materials, principally silicon, germanium, and gallium arsenide, as well as organic semiconductors. Semiconductor devices have replaced thermionic devices in most applications...
diode
Diode
In electronics, a diode is a type of two-terminal electronic component with a nonlinear current–voltage characteristic. A semiconductor diode, the most common type today, is a crystalline piece of semiconductor material connected to two electrical terminals...
, the cathode is the N–doped layer of the PN junction with a high density of free electrons as a result of doping, and an equal density of fixed positive charges, which are the dopants that have been thermally ionized. In the anode, the converse applies: It features a high density of free "holes" and consequently fixed negative dopants which have captured an electron (hence the origin of the holes).
When P and N-doped layers are placed in contact, diffusion ensures that electrons flow from high to low density areas: That is, from the N to the P side. They leave behind the fixed positively charged dopants near the junction. Similarly, holes diffuse from P to N leaving behind fixed negative ionised dopants near the junction. These layers of fixed positive and negative charges, collectively known as the depletion layer because they are depleted of free electrons and holes. The depletion layer at the junction is at the origin of the diode's rectifying properties. This is due to the resulting internal field and corresponding potential barrier which inhibit current flow in reverse applied bias which increases the internal depletion layer field. Conversely, they allow it in forwards applied bias where the applied bias reduces the built in potential barrier.
Electrons which diffuse from the cathode into the P-doped layer, or anode, become what is termed "minority carriers" and tend to recombine there with the majority carriers, which are holes, on a timescale characteristic of the material which is the p-type minority carrier lifetime. Similarly, holes diffusing into the N-doped layer become minority carriers and tend to recombine with electrons. In equilibrium, with no applied bias, thermally assisted diffusion of electrons and holes in opposite directions across the depletion layer ensure a zero net current with electrons flowing from cathode to anode and recombining, and holes flowing from anode to cathode across the junction or depletion layer and recombining.
Like a typical diode, there is a fixed anode and cathode in a Zener diode, but it will conduct current in the reverse direction (electrons from anode to cathode) if its breakdown voltage or "Zener voltage" is exceeded.
See also
- AnodeAnodeAn anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
- BatteryBattery (electricity)An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy. Since the invention of the first battery in 1800 by Alessandro Volta and especially since the technically improved Daniell cell in 1836, batteries have become a common power...
- Cathode biasCathode biasIn order for a vacuum tube to operate in a fairly linear region of its characteristic curve, the grid element must be maintained at a bias voltage more negative than the cathode. One method for accomplishing this is "cathode bias."-Early techniques:...
- ElectrodeElectrodeAn electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit...
- ElectrolysisElectrolysisIn chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
- Electrolytic cellElectrolytic cellAn electrolytic cell decomposes chemical compounds by means of electrical energy, in a process called electrolysis; the Greek word lysis means to break up. The result is that the chemical energy is increased...
- Electron tubeElectron tubeElectron tube can be used to describe either of two things:* Vacuum tube* Gas-filled tube...
- Oxidation-reduction
- PEDOTPoly(3,4-ethylenedioxythiophene)Poly or PEDOT is a conducting polymer based on 3,4-ethylenedioxylthiophene or EDOT monomer. Advantages of this polymer are optical transparency in its conducting state, high stability and moderate band gap and low redox potential...

