
Carbonic anhydrase
Encyclopedia
The carbonic anhydrases (or carbonate dehydratases) form a family of enzyme
s that catalyze the rapid interconversion of carbon dioxide
and water
to bicarbonate
and protons (or vice-versa), a reversible reaction
that occurs rather slowly in the absence of a catalyst. The active site
of most carbonic anhydrases contains a zinc
ion; they are therefore classified as metalloenzymes
.
One of the functions of the enzyme in animals is to interconvert carbon dioxide and bicarbonate to maintain acid-base balance in blood and other tissues, and to help transport carbon dioxide out of tissues.
 (in tissue
(in tissue
s - high CO2 concentration)
The reaction rate
of carbonic anhydrase is one of the fastest of all enzymes, and its rate is typically limited by the diffusion
rate of its substrate
s.
Typical catalytic rates of the different forms of this enzyme ranging between 104 and 106 reactions per second.
The reverse reaction is also relatively slow (kinetics in the 15-second range), which is why a carbonated drink does not instantly degas when opening the container. However it will rapidly degas in the mouth when it comes in contact with carbonic anhydrase that is contained in saliva.
An anhydrase is defined as an enzyme that catalyzes the removal of a water molecule from a compound, and so it is this "reverse" reaction that gives carbonic anhydrase its name, because it removes a water molecule from carbonic acid.
 (in lung
(in lung
s and nephrons of the kidney
- low CO2 concentration, in plant cells)
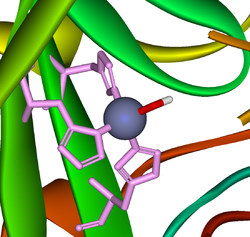 A zinc
A zinc
prosthetic group in the enzyme is coordinated in three positions by histidine side-chains. The fourth coordination position is occupied by water. This causes polarisation of the hydrogen-oxygen bond, making the oxygen slightly more negative, thereby weakening the bond.
A fourth histidine is placed close to the substrate of water and accepts a proton
, in an example of general acid-general base catalysis. This leaves a hydroxide
attached to the zinc.
The active site also contains specificity pocket for carbon dioxide, bringing it close to the hydroxide group. This allows the electron rich hydroxide to attack the carbon dioxide, forming bicarbonate.
 There are at least five distinct CA families (α, β, γ, δ and ε). These families have no significant amino acid sequence similarity and in most cases are thought to be an example of convergent evolution
There are at least five distinct CA families (α, β, γ, δ and ε). These families have no significant amino acid sequence similarity and in most cases are thought to be an example of convergent evolution
. The α-CAs are found in humans.
s are divided into four broad subgroups, which, in turn consist of several isoforms:
There are three additional "acatalytic" CA isoforms (CA-VIII, CA-X, and CA-XI) whose functions remain unclear.
and plant chloroplast
CAs belong to the beta family.
Two signature pattern
s for this family have been identified:
that grow in hot springs.
s. The distinction of this class of CA has recently come into question, however.
in a few chemolithotrophs and marine cyanobacteria that contain cso-carboxysome
s. Recent 3-dimensional analyses suggest that ε-CA bears some structural resemblance to β-CA, particularly near the metal ion site. Thus, the two forms may be distantly related, even though the underlying amino acid sequence has since diverged considerably.
rings of 3 histidine
residues, His94, His96, and His119.
The primary function of the enzyme in animals is to interconvert carbon dioxide and bicarbonate to maintain acid-base balance in blood and other tissues, and to help transport carbon dioxide out of tissues.
There exist at least 14 different isoforms in mammals. Plant
s contain a different form called β-carbonic anhydrase, which, from an evolutionary standpoint, is a distinct enzyme, but participates in the same reaction and also uses a zinc ion in its active site. In plants, carbonic anhydrase helps raise the concentration of CO2 within the chloroplast
in order to increase the carboxylation rate of the enzyme RuBisCO
. This is the reaction that integrates CO2 into organic carbon sugars during photosynthesis
, and can use only the CO2 form of carbon, not carbonic acid or bicarbonate.
A cadmium
-containing carbonic anhydrase was found to be expressed in marine diatom
s during zinc limitation. In the open ocean, zinc is often in such low concentrations that it can limit the growth of phytoplankton
like diatoms; thus, a carbonic anhydrase using a different metal ion would be beneficial in these environments. Cadmium
has in general been thought of as a very toxic heavy metal
without biological function. This peculiar carbonic anhydrase form hosts the only known beneficial cadmium-dependent biological reaction.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s that catalyze the rapid interconversion of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
to bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
and protons (or vice-versa), a reversible reaction
Reversible reaction
A reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
that occurs rather slowly in the absence of a catalyst. The active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
of most carbonic anhydrases contains a zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
ion; they are therefore classified as metalloenzymes
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. Indeed, about one quarter to one third of all proteins require metals to...
.
One of the functions of the enzyme in animals is to interconvert carbon dioxide and bicarbonate to maintain acid-base balance in blood and other tissues, and to help transport carbon dioxide out of tissues.
Reaction
The reaction catalyzed by carbonic anhydrase is: (in tissue
(in tissueBiological tissue
Tissue is a cellular organizational level intermediate between cells and a complete organism. A tissue is an ensemble of cells, not necessarily identical, but from the same origin, that together carry out a specific function. These are called tissues because of their identical functioning...
s - high CO2 concentration)
The reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
of carbonic anhydrase is one of the fastest of all enzymes, and its rate is typically limited by the diffusion
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
rate of its substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
s.
Typical catalytic rates of the different forms of this enzyme ranging between 104 and 106 reactions per second.
The reverse reaction is also relatively slow (kinetics in the 15-second range), which is why a carbonated drink does not instantly degas when opening the container. However it will rapidly degas in the mouth when it comes in contact with carbonic anhydrase that is contained in saliva.
An anhydrase is defined as an enzyme that catalyzes the removal of a water molecule from a compound, and so it is this "reverse" reaction that gives carbonic anhydrase its name, because it removes a water molecule from carbonic acid.
 (in lung
(in lungLung
The lung is the essential respiration organ in many air-breathing animals, including most tetrapods, a few fish and a few snails. In mammals and the more complex life forms, the two lungs are located near the backbone on either side of the heart...
s and nephrons of the kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
- low CO2 concentration, in plant cells)
Mechanism

Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
prosthetic group in the enzyme is coordinated in three positions by histidine side-chains. The fourth coordination position is occupied by water. This causes polarisation of the hydrogen-oxygen bond, making the oxygen slightly more negative, thereby weakening the bond.
A fourth histidine is placed close to the substrate of water and accepts a proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
, in an example of general acid-general base catalysis. This leaves a hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
attached to the zinc.
The active site also contains specificity pocket for carbon dioxide, bringing it close to the hydroxide group. This allows the electron rich hydroxide to attack the carbon dioxide, forming bicarbonate.
CA families

Convergent evolution
Convergent evolution describes the acquisition of the same biological trait in unrelated lineages.The wing is a classic example of convergent evolution in action. Although their last common ancestor did not have wings, both birds and bats do, and are capable of powered flight. The wings are...
. The α-CAs are found in humans.
α-CA
The CA enzymes found in mammalMammal
Mammals are members of a class of air-breathing vertebrate animals characterised by the possession of endothermy, hair, three middle ear bones, and mammary glands functional in mothers with young...
s are divided into four broad subgroups, which, in turn consist of several isoforms:
- the cytosolCytosolThe cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
ic CAs (CA-I, CA-IICarbonic anhydrase IICarbonic anhydrase II , is one of fourteen forms of human α carbonic anhydrases. Carbonic anhydrase catalyzes reversible hydration of carbon dioxide...
, CA-III, CA-VII and CA XIII) - mitochondrialMitochondrionIn cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
CAs (CA-VA and CA-VB) - secreted CAs (CA-VI)
- membrane-associated CAs (CA-IV, CA-IX, CA-XII, CA-XIV and CA-XV)
There are three additional "acatalytic" CA isoforms (CA-VIII, CA-X, and CA-XI) whose functions remain unclear.
| Isoform | Gene | Molecular mass Molecular mass The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u... |
Location (cell) | Location (tissue) | Specific activity of human enzymes (except for mouse CA XV) (s−1) | Sensitivity to sulfonamides (acetazolamide in this table) KI (nM) |
|---|---|---|---|---|---|---|
| CA-I | 29 kDa | cytosol Cytosol The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments.... |
red blood cell Red blood cell Red blood cells are the most common type of blood cell and the vertebrate organism's principal means of delivering oxygen to the body tissues via the blood flow through the circulatory system... and GI tract Gastrointestinal tract The human gastrointestinal tract refers to the stomach and intestine, and sometimes to all the structures from the mouth to the anus. .... |
2.0 × 105 | 250 | |
| CA-II Carbonic anhydrase II Carbonic anhydrase II , is one of fourteen forms of human α carbonic anhydrases. Carbonic anhydrase catalyzes reversible hydration of carbon dioxide... |
29 kDa | cytosol Cytosol The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments.... |
almost ubiquitous | 1.4 × 106 | 12 | |
| CA-III | 29 kDa | cytosol Cytosol The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments.... |
8% of soluble protein in Type I muscle Muscle Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to... |
1.3 × 104 | 240000 | |
| CA-IV | 35 kDa | extracellular GPI Glycophosphatidylinositol Glycosylphosphatidylinositol is a glycolipid that can be attached to the C-terminus of a protein during posttranslational modification... -linked |
GI tract Gastrointestinal tract The human gastrointestinal tract refers to the stomach and intestine, and sometimes to all the structures from the mouth to the anus. .... , kidney Kidney The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and... , endothelium |
1.1 × 106 | 74 | |
| CA-VA | 34.7 kDa (predicted) | mitochondria | liver | 2.9 × 105 | 63 | |
| CA-VB | 36.4 kDa (predicted) | mitochondria | widely distributed | 9.5 × 105 | 54 | |
| CA-VI | 39-42 kDa | secretory | saliva and milk | 3.4 × 105 | 11 | |
| CA-VII | 29 kDa | cytosol Cytosol The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments.... |
widely distributed | 9.5 × 105 | 2.5 | |
| CA-IX | 54, 58 kDa | cell membrane Cell membrane The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell... -associated |
normal GI tract Gastrointestinal tract The human gastrointestinal tract refers to the stomach and intestine, and sometimes to all the structures from the mouth to the anus. .... , several cancers |
1.1 × 106 | 16 | |
| CA-XII | 44 kDa | extracellularily located active site Active site In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that... |
kidney Kidney The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and... , certain cancer Cancer Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the... s |
4.2 × 105 | 5.7 | |
| CA-XIII | 29 kDa | cytosol Cytosol The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments.... |
widely distributed | 1.5 × 105 | 16 | |
| CA-XIV | 54 kDa | extracellularily located active site Active site In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that... |
kidney Kidney The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and... , heart Heart The heart is a myogenic muscular organ found in all animals with a circulatory system , that is responsible for pumping blood throughout the blood vessels by repeated, rhythmic contractions... , skeletal muscle Skeletal muscle Skeletal muscle is a form of striated muscle tissue existing under control of the somatic nervous system- i.e. it is voluntarily controlled. It is one of three major muscle types, the others being cardiac and smooth muscle... , brain Brain The brain is the center of the nervous system in all vertebrate and most invertebrate animals—only a few primitive invertebrates such as sponges, jellyfish, sea squirts and starfishes do not have one. It is located in the head, usually close to primary sensory apparatus such as vision, hearing,... |
3.1 × 105 | 41 | |
| CA-XV | 34-36 kDa | extracellular GPI Glycophosphatidylinositol Glycosylphosphatidylinositol is a glycolipid that can be attached to the C-terminus of a protein during posttranslational modification... -linked |
kidney Kidney The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and... , not expressed in human tissues |
4.7 × 105 | 72 |
β-CA
Most prokaryoticProkaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
and plant chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
CAs belong to the beta family.
Two signature pattern
Sequence motif
In genetics, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and has, or is conjectured to have, a biological significance...
s for this family have been identified:
- C-[SA]-D-S-R-[LIVM]-x-[AP]
- [EQ]-[YF]-A-[LIVM]-x(2)-[LIVM]-x(4)-[LIVMF](3)-x-G-H-x(2)-C-G
γ-CA
The gamma class of CAs come from methane-producing bacteriaMethanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in anoxic conditions. They are classified as archaea, a group quite distinct from bacteria...
that grow in hot springs.
δ-CA
The delta class of CAs has been described in diatomDiatom
Diatoms are a major group of algae, and are one of the most common types of phytoplankton. Most diatoms are unicellular, although they can exist as colonies in the shape of filaments or ribbons , fans , zigzags , or stellate colonies . Diatoms are producers within the food chain...
s. The distinction of this class of CA has recently come into question, however.
ε-CA
The epsilon class of CAs occurs exclusively in bacteriaBacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
in a few chemolithotrophs and marine cyanobacteria that contain cso-carboxysome
Carboxysome
Carboxysomes are bacterial microcompartments that contain enzymes involved in carbon fixation. Carboxysomes are made of polyhedral protein shells about 80 to 140 nanometres in diameter. These compartments are thought to concentrate carbon dioxide to overcome the inefficiency of RuBisCo - the...
s. Recent 3-dimensional analyses suggest that ε-CA bears some structural resemblance to β-CA, particularly near the metal ion site. Thus, the two forms may be distantly related, even though the underlying amino acid sequence has since diverged considerably.
Structure and function of carbonic anhydrase
Several forms of carbonic anhydrase occur in nature. In the best-studied α-carbonic anhydrase form present in animals, the zinc ion is coordinated by the imidazoleImidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
rings of 3 histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residues, His94, His96, and His119.
The primary function of the enzyme in animals is to interconvert carbon dioxide and bicarbonate to maintain acid-base balance in blood and other tissues, and to help transport carbon dioxide out of tissues.
There exist at least 14 different isoforms in mammals. Plant
Plant
Plants are living organisms belonging to the kingdom Plantae. Precise definitions of the kingdom vary, but as the term is used here, plants include familiar organisms such as trees, flowers, herbs, bushes, grasses, vines, ferns, mosses, and green algae. The group is also called green plants or...
s contain a different form called β-carbonic anhydrase, which, from an evolutionary standpoint, is a distinct enzyme, but participates in the same reaction and also uses a zinc ion in its active site. In plants, carbonic anhydrase helps raise the concentration of CO2 within the chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
in order to increase the carboxylation rate of the enzyme RuBisCO
RuBisCO
Ribulose-1,5-bisphosphate carboxylase oxygenase, commonly known by the shorter name RuBisCO, is an enzyme involved in the first major step of carbon fixation, a process by which atmospheric carbon dioxide is converted by plants to energy-rich molecules such as glucose. RuBisCo is an abbreviation...
. This is the reaction that integrates CO2 into organic carbon sugars during photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
, and can use only the CO2 form of carbon, not carbonic acid or bicarbonate.
A cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
-containing carbonic anhydrase was found to be expressed in marine diatom
Diatom
Diatoms are a major group of algae, and are one of the most common types of phytoplankton. Most diatoms are unicellular, although they can exist as colonies in the shape of filaments or ribbons , fans , zigzags , or stellate colonies . Diatoms are producers within the food chain...
s during zinc limitation. In the open ocean, zinc is often in such low concentrations that it can limit the growth of phytoplankton
Phytoplankton
Phytoplankton are the autotrophic component of the plankton community. The name comes from the Greek words φυτόν , meaning "plant", and πλαγκτός , meaning "wanderer" or "drifter". Most phytoplankton are too small to be individually seen with the unaided eye...
like diatoms; thus, a carbonic anhydrase using a different metal ion would be beneficial in these environments. Cadmium
Cadmium
Cadmium is a chemical element with the symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Similar to zinc, it prefers oxidation state +2 in most of its compounds and similar to mercury it shows a low...
has in general been thought of as a very toxic heavy metal
Heavy metals
A heavy metal is a member of a loosely-defined subset of elements that exhibit metallic properties. It mainly includes the transition metals, some metalloids, lanthanides, and actinides. Many different definitions have been proposed—some based on density, some on atomic number or atomic weight,...
without biological function. This peculiar carbonic anhydrase form hosts the only known beneficial cadmium-dependent biological reaction.

