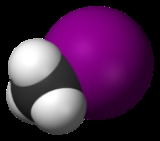
Iodine
Encyclopedia
Iodine is a chemical element
with the symbol I and atomic number
53. The name is pronounced icon , ˈ , or (British English) ˈ . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.
Iodine and its compounds are primarily used in nutrition
, and industrially in the production of acetic acid
and certain polymers. Iodine's relatively high atomic number, low toxicity, and ease of attachment to organic compounds have made it a part of many X-ray contrast
materials in modern medicine. Iodine has only one stable isotope
. A number of iodine radioisotopes are also used in medical applications.
Iodine is found on Earth mainly as the highly water-soluble iodide I-, which concentrates it in oceans and brine pools. Like the other halogen
s, free iodine occurs mainly as a diatomic
molecule I2, and then only momentarily after being oxidized from iodide by an oxidant like free oxygen. In the universe and on Earth, iodine's high atomic number makes it a relatively rare element
, ranking 47th in abundance. However, its presence in ocean water has given it a role in biology. It is the heaviest essential element utilized widely by life in biological functions (only tungsten
, employed in enzymes by a few species of bacteria, is heavier). Iodine's rarity in many soils, due to initial low abundance as a crust-element, and also leaching of soluble iodide by rainwater, has led to many deficiency problems in land animals and inland human populations. Iodine deficiency
affects about two billion people and is the leading preventable cause of intellectual disabilities.
Iodine is required by higher animals, which use it to synthesize thyroid hormones, which contain the element. Because of this function, radioisotopes of iodine are concentrated in the thyroid gland along with nonradioactive iodine. The radioisotope iodine-131
, which has a high fission product yield
, concentrates in the thyroid, and is one of the most carcinogenic of nuclear fission
products.
Elemental iodine dissolves easily in most organic solvents such as hexane
or chloroform
owing to its lack of polarity, but is only slightly soluble in water. However, the solubility of elemental iodine in water can be increased by the addition of potassium iodide
. The molecular iodine reacts reversibly with the negative ion, generating the triiodide
anion I3− in equilibrium
, which is soluble in water. This is also the formulation of some types of medicinal (antiseptic) iodine, although tincture of iodine
classically dissolves the element in aqueous ethanol
.
The colour of solutions of elemental iodine change depends on the polarity of the solvent. In non-polar solvents like hexane, solution are violet; in moderately polar dichloromethane
, the solution is dark crimson, and, in strongly polar solvents such as acetone
or ethanol, it appears orange or brown. This effect is due to the formation of adducts.
Iodine melts at the relatively low temperature of 113.7 °C, although the liquid is often obscured by a dense violet vapor of gaseous iodine.
 Iodine is rare in the solar system
Iodine is rare in the solar system
and Earth's crust (47th in abundance); however, iodide
salts are often very soluble
in water. Iodine occurs in slightly greater concentrations in seawater
than in rocks, 0.05 vs 0.04 ppm. Minerals containing iodine include caliche
, found in Chile
. The brown algae
Laminaria
and Fucus
found in temperate zones of the Northern Hemisphere contain 0.028–0.454 dry weight percent of iodine. Aside from tungsten
, iodine is the heaviest element to be essential in living organisms, and iodine is the heaviest element thought to be needed by higher animals. About 19,000 tonne
s are produced annually from natural sources.
Organoiodine compound
s are produced by marine life forms, the most notable being iodomethane (commonly called methyl iodide). The total iodomethane that is produced by the marine environment, by microbial activity in rice paddies and by the burning of biological material is estimated to be 214 kilotonnes/year. The volatile iodomethane is broken up in the atmosphere as part of a global iodine cycle.
force called the London Forces
, and this interaction is responsible for the higher melting point compared to more compact halogens, which are also diatomic. Since the atomic size of Iodine is larger, its melting point is higher. The solid crystallizes as orthorhombic crystals. The crystal motif in the Hermann–Mauguin notation is Cmca (No 64), Pearson symbol
oS8. The I-I bond is relatively weak, with a bond dissociation energy
of 36 kcal/mol. In fact, most bonds to iodine tend to be weaker than for the lighter halides. One consequence of this weak bonding is the relatively high tendency of I2 molecules to dissociate into atomic iodine.
, found in Chile
, and the iodine-containing brines of gas and oil fields, especially in Japan and the United States. The caliche, found in Chile, contains sodium nitrate
, which is the main product of the mining activities and small amounts of sodium iodate and sodium iodide. In the extraction of sodium nitrate, the sodium iodate and sodium iodide are extracted. The high concentration of iodine in the caliche and the extensive mining made Chile the largest producer of iodine in 2007.
Most other producers use natural occurring brine for the production of iodine. The Japanese Minami Kanto gas field
east of Tokyo
and the American Anadarko Basin
gas field in northwest Oklahoma
are the two largest sources for iodine from brine. The brine has a temperature of over 60°C owing to the depth of the source. The brine
is first purified and acidified using sulfuric acid
, then the iodide present is oxidized to iodine with chlorine
. An iodine solution is produced, but is dilute and must be concentrated. Air is blown into the solution, causing the iodine to evaporate, then it is passed into an absorbing tower containing acid where sulfur dioxide
is added to reduce
the iodine. The hydrogen iodide
(HI) is reacted with chlorine to precipitate the iodine. After filtering and purification the iodine is packed.
The production of iodine from seawater via electrolysis
is not used owing to the sufficient abundance of iodine-rich brine. Another source of iodine was kelp
, used in the 18th and 19th centuries, but it is no longer economically viable.
 Commercial samples often contain high concentrations of impurities, which can be removed by sublimation. The element may also be prepared in an ultra-pure form through the reaction of potassium iodide
Commercial samples often contain high concentrations of impurities, which can be removed by sublimation. The element may also be prepared in an ultra-pure form through the reaction of potassium iodide
with copper(II) sulfate, which gives copper(II) iodide initially. That decomposes spontaneously to copper(I) iodide
and iodine:
There are also other methods of isolating this element in the laboratory, for example, the method used to isolate other halogens: Oxidation of the iodide in hydrogen iodide
(often made in situ with an iodide and sulfuric acid) by manganese dioxide (see below in Descriptive chemistry).
s of iodine, only one, 127I, is stable.
The longest-lived radioisotope, 129I, has a half-life of 15.7 million years. This is long enough to make it a permanent fixture of the environment on human time scales, but far too short for it to exist as a primordial isotope today. Instead, iodine-129
is an extinct radionuclide
, and its presence in the early solar system is inferred from the observation of an excess of its daughter xenon-129. This nuclide is also newly-made by cosmic rays and as a byproduct of human nuclear fission, which it is used to monitor as a very long-lived environmental contaminant.
The next-longest-lived radioisotope, iodine-125
, has a half-life of 59 days. It is used as a convenient gamma-emitting tag for proteins in biological assays, and a few nuclear medicine
imaging tests where a longer half-life is required. It is also commonly used in brachytherapy
implanted capsules, which kill tumors by local short-range gamma radiation (but where the isotope is never released into the body).
Iodine-123
(half-life 13 hours) is the isotope of choice for nuclear medicine imaging of the thyroid gland, which naturally accumulates all iodine isotopes.
Iodine-131
(half-life 8 days) is a beta-emitting isotope, which is a common nuclear fission product. It is preferably administered to humans only in very high doses which destroy all tissues that accumulate it (usually the thyroid), which in turn prevents these tissues from developing cancer from a lower dose (paradoxically, a high dose of this isotope appears safer for the thryoid than a low dose). Like other radioiodines, I-131 accumulates in the thyroid gland, but unlike the others, in small amounts it is highly carcinogenic there, it seems, owing to the high local cell mutation due to damage from beta decay
. Because of this tendency of 131I to cause high damage to cells that accumulate it and other cells near them (0.6 to 2 mm away, the range of the beta rays), it is the only iodine radioisotope used as direct therapy, to kill tissues such as cancers that take up artificially iodinated molecules (example, the compound iobenguane
, also known as MIBG). For the same reason, only the iodine isotope I-131 is used to treat Grave's disease and those types of thyroid cancers (sometimes in metastatic form) where the tissue that requires destruction, still functions to naturally accumulate iodide.
Nonradioactive ordinary potassium iodide
(iodine-127), in a number of convenient forms (tablets or solution) may be used to saturate the thyroid gland's ability to take up further iodine, and thus protect against accidental contamination from iodine-131 generated by nuclear fission
accidents, such as the Chernobyl disaster
and more recently the Fukushima I nuclear accidents, as well as from contamination from this isotope in nuclear fallout
from nuclear weapon
s.
in 1811. He was born to a manufacturer of saltpeter
(a vital part of gunpowder
). At the time of the Napoleonic Wars
, France
was at war and saltpeter was in great demand. Saltpeter produced from French niter
beds required sodium carbonate
, which could be isolated from seaweed
collected on the coasts of Normandy
and Brittany
. To isolate the sodium carbonate, seaweed was burned and the ash washed with water. The remaining waste was destroyed by adding sulfuric acid
. Courtois once added excessive sulfuric acid and a cloud of purple vapor rose. He noted that the vapor crystallized on cold surfaces, making dark crystals. Courtois suspected that this was a new element but lacked funding to pursue it further.
Courtois gave samples to his friends, Charles Bernard Desormes
(1777–1862) and Nicolas Clément (1779–1841), to continue research. He also gave some of the substance to chemist
Joseph Louis Gay-Lussac
(1778–1850), and to physicist
André-Marie Ampère
(1775–1836). On 29 November 1813, Dersormes and Clément made public Courtois's discovery. They described the substance to a meeting of the Imperial Institute of France. On December 6, Gay-Lussac announced that the new substance was either an element or a compound of oxygen. It was Gay-Lussac who suggested the name "iode", from the Greek word ιώδες (iodes) for violet (because of the color of iodine vapor). Ampère had given some of his sample to Humphry Davy
(1778–1829). Davy did some experiments on the substance and noted its similarity to chlorine
. Davy sent a letter dated December 10 to the Royal Society of London stating that he had identified a new element. Arguments erupted between Davy and Gay-Lussac over who identified iodine first, but both scientists acknowledged Courtois as the first to isolate the element.
by the Monsanto
and Cativa process
es. In these technologies, which support the world's demand for acetic acid, hydroiodic acid converts the methanol
feedstock into methyl iodide, which undergoes carbonylation
. Hydrolysis of the resulting acetyl iodide regenerates hydroiodic acid and gives acetic acid.
anion I3- generated in situ by adding iodide
to poorly water-soluble elemental iodine (the reverse chemical reaction makes some free elemental iodine available for antisepsis). In alternative fashion, iodine may come from iodophor
s, which contain iodine complexed with a solubilizing agent (iodide ion may be thought of loosely as the iodophor in triiodide water solutions). Examples of such preparations include:
Potassium iodide has been used as an expectorant, although this use is increasingly uncommon. In medicine, potassium iodide
is used to treat acute thyrotoxicosis, usually as a saturated solution of potassium iodide (SSKI). It is also used to block uptake of iodine-131
in the thyroid gland (see isotopes section above), when this isotope is used as part of radiopharmaceuticals (such as iobenguane
) that are not targetted to the thyroid or thyroid type tissues.
Iodine-131 (in the chemical form of iodide) is a component of nuclear fallout
and a particularly dangerous one owing to the thyroid gland's propensity to concentrate ingested iodine, where it is kept for periods longer than this isotope's radiological half-life of eight days. For this reason, if people are expected to be exposed to a significant amount of environmental radioactive iodine (iodine-131 in fallout), they may be instructed to take non-radioactive potassium iodide tablets. The typical adult dose is one 130 mg tablet per 24 hours, supplying 100 mg (100,000 micrograms) iodine, as iodide ion. (Note: typical daily dose of iodine to maintain normal health is of order 100 micrograms; see "Dietary Intake" below.) By ingesting this large amount of non-radioactive iodine, radioactive iodine uptake by the thyroid gland is minimized. See the main article above for more on this topic.
 Iodine, as a heavy element, is quite radio-opaque. Organic compounds of a certain type (typically iodine-substituted benzene derivatives) are, thus, used in medicine
Iodine, as a heavy element, is quite radio-opaque. Organic compounds of a certain type (typically iodine-substituted benzene derivatives) are, thus, used in medicine
as X-ray radiocontrast
agents for intravenous injection. This is often in conjunction with advanced X-ray techniques such as angiography and CT scanning. At present, all water-soluble radiocontrast agents rely on iodine.
.
The organoiodine compound erythrosine
is an important food coloring agent. Perfluoroalkyl iodides are precursors to important surfactants, such as perfluorooctanesulfonic acid.
s.
(20.5 g/100 ml at 15 °C, 21.43 g/100 ml at 25 °C), diethyl ether
(20.6 g/100 ml at 17 °C, 25.20 g/100 ml at 25 °C), chloroform
, acetic acid
, glycerol
, benzene
(14.09 g/100 ml at 25 °C), carbon tetrachloride
(2.603 g/100 ml at 35 °C), and carbon disulfide
(16.47 g/100 ml at 25 °C). Elemental iodine is poorly soluble in water, with one gram dissolving in 3450 ml at 20 °C and 1280 ml at 50 °C. Aqueous and ethanol solutions are brown reflecting the role of these solvents as Lewis bases. Solutions in chloroform, carbon tetrachloride, and carbon disulfide are violet, the color of iodine vapor.
One of the most distinctive properties of iodine is the way that its solubility in water is enhanced by the presence of iodide ions. The dissolution of iodine in aqueous solutions containing iodide (e.g., from hydroiodic acid, potassium iodide
, etc.) results from the formation of the I3−
ion. Dissolved bromide
s also improve water solubility of iodine.
Iodine is easily oxidized and easily reduced. Most common is the interconversion of I- and I2. Molecular iodine can be prepared by oxidizing iodide
s with chlorine:
or with manganese dioxide in acid solution:
Iodine is reduced to hydroiodic acid by hydrogen sulfide
and hydrazine
:
When dissolved in fuming sulfuric acid
(65% oleum), iodine forms an intense blue solution. The blue color is due to cation, the result of iodine being oxidized by :
The cation is also formed in the oxidation of iodine by
or . The resulting or can be isolated as deep blue crystals. The solutions of these salts turn red when cooled below −60°C, owing to the formation of the cation:
Under slightly more alkaline conditions, disproportionates into and an iodine(III) compound. Excess iodine can then react with to form (green) and (black).
.
By contrast with chlorine
, the formation of the hypohalite ion (IO–) in neutral aqueous solutions of iodine is negligible.
Organic derivatives of hypoiodate (2-Iodoxybenzoic acid
, and Dess-Martin periodinane
) are used in organic chemistry.
Iodic acid
(HIO3), periodic acid
(HIO4) and their salts are strong oxidizers and are of some use in organic synthesis
. Iodine is oxidized to iodate
by nitric acid
as well as by chlorate
s:
for the production of acetic acid. Titanium and aluminium iodides are used in the production of butadiene, a precursor to rubber tires.
Alkali metal salts are common colourless solids that are highly soluble in water. Potassium iodide
is a convenient source of the iodide
anion; it is easier to handle than sodium iodide
because it is not hygroscopic. Both salts are mainly used in the production of iodized salt. Sodium iodide is especially useful in the Finkelstein reaction
, because it is soluble in acetone
, whereas potassium iodide is less so. In this reaction, an alkyl chloride is converted to an alkyl iodide. This relies on the insolubility of sodium chloride
in acetone to drive the reaction:
Despite having the lowest electronegativity of the common halogens, iodine reacts violently with some metals, such as aluminium:
This reaction produces 314 kJ per mole of aluminum, comparable to thermite's 425 kJ. Yet the reaction initiates spontaneously, and if unconfined, causes a cloud of gaseous iodine due to the high temperature.
and trichloride
; iodine pentafluoride
and heptafluoride
.
, approved as a soil fumigant. Iodinated organic compounds are used as synthetic reagents.
, red phosphorus, and iodine. The iodinating reagent is phosphorus triiodide
that is formed in situ:
The iodoform test uses an alkaline solution of iodine to react with methyl ketones to give the labile triiodomethide leaving group, forming iodoform, which precipitates.
Aryl and alkyl iodides both form Grignard reagents. Iodine is sometimes used to activate magnesium when preparing Grignard reagents. Alkyl iodides such as iodomethane are good alkylating agents. Some drawbacks to use of organoiodine compounds in chemical synthesis are:
 Iodine is a common general stain used in thin-layer chromatography. In particular, iodine forms an intense blue complex with the glucose polymers starch
Iodine is a common general stain used in thin-layer chromatography. In particular, iodine forms an intense blue complex with the glucose polymers starch
and glycogen
. Several analytical methods rely on this property:
.
for life, the heaviest element commonly needed by living organisms. Only tungsten
, a component of a few bacterial enzymes, has a higher atomic number and atomic weight.
Iodine's main role in animal biology is as a constituent of the thyroid
hormone
s thyroxine
(T4) and triiodothyronine
(T3). These are made from addition condensation products of the amino acid tyrosine
, and are stored prior to release in an iodine-containing protein
called thyroglobulin
. T4 and T3 contain four and three atoms of iodine per molecule
, respectively. The thyroid gland actively absorbs iodide from the blood
to make and release these hormones into the blood, actions that are regulated by a second hormone TSH
from the pituitary. Thyroid hormones are phylogenetically very old molecules that are synthesized by most multicellular organisms, and that even have some effect on unicellular organisms.
Thyroid hormones play a basic role in biology, acting on gene transcription to regulate the basal metabolic rate
. The total deficiency of thyroid hormones can reduce basal metabolic rate up to 50%, while in excessive production of thyroid hormones the basal metabolic rate can be increased by 100%. T4 acts largely as a precursor to T3, which is (with minor exceptions) the biologically active hormone.
Iodine has a nutritional relationship with selenium
. A family of selenium-dependent enzymes called deiodinase
s converts T4 to T3 (the active hormone) by removing an iodine atom from the outer tyrosine ring. These enzymes also convert T4 to reverse T3 (rT3) by removing an inner ring iodine atom, and convert T3 to 3,3'-diiodothyronine
(T2) also by removing an inner ring atom. Both of the latter are inactivated hormones that are ready for disposal and have, in essence, no biological effects. A family of non-selenium-dependent enzymes then further deiodinates the products of these reactions.
Iodine accounts for 65% of the molecular weight of T4 and 59% of the T3. Fifteen to 20 mg of iodine is concentrated in thyroid tissue and hormones, but 70% of the body's iodine is distributed in other tissues, including mammary glands, eyes, gastric mucosa, the cervix, and salivary glands. In the cells of these tissues, iodide enters directly by sodium-iodide symporter
(NIS). Its role in mammary tissue is related to fetal and neonatal development, but its role in the other tissues is unknown.
recommended by the United States Institute of Medicine
is between 110 and 130 µg
for infants up to 12 months, 90 µg for children up to eight years, 130 µg for children up to 13 years, 150 µg for adults, 220 µg for pregnant women and 290 µg for lactating mothers
. The Tolerable Upper Intake Level (UL) for adults is 1,100 μg/day (1.1 mg/day). The tolerable upper limit was assessed by analyzing the effect of supplementation on thyroid-stimulating hormone
.
The thyroid gland needs no more than 70 micrograms /day to synthesize the requisite daily amounts of T4 and T3. The higher recommended daily allowance levels of iodine seem necessary for optimal function of a number of body systems, including lactating breast, gastric mucosa, salivary glands, oral mucosa, thymus, epidermis, choroid plexus, etc. The high iodide-concentration of thymus tissue in particular suggests an anatomical rationale for this role of iodine in the immune system. The trophic, antioxidant and apoptosis-inductor actions and the presumed anti-tumour activity of iodides has been suggested to also be important for prevention of oral and salivary glands diseases.
Natural sources of iodine include sea life, such as kelp
and certain seafood, as well as plants grown on iodine-rich soil. Iodized salt is fortified with iodine.
As of 2000, the median intake of iodine from food in the United States was 240 to 300 μg/day for men and 190 to 210 μg/day for women. In Japan, consumption is much higher, owing to the frequent consumption of seaweed or kombu
kelp.
After iodine fortification programs (e.g., iodized salt) have been implemented, some cases of iodine-induced hyperthyroidism
have been observed (so called Jod-Basedow phenomenon). The condition seems to occur mainly in people over forty, and the risk appears higher when iodine deficiency is severe and the initial rise in iodine intake is high.
gives rise to hypothyroidism, symptoms of which are extreme fatigue, goitre
, mental slowing, depression, weight gain, and low basal body temperatures. Iodine deficiency is the leading cause of preventable mental retardation
, a result that occurs primarily when babies or small children are rendered hypothyroidic by a lack of the element. The addition of iodine to table salt has largely eliminated this problem in the wealthier nations, but, as of March 2006, iodine deficiency remained a serious public health problem in the developing world. Iodine deficiency is also a problem in certain areas of Europe.
Other possible health effects being investigated as being related to deficiency include:
and Lugol's solution are capable of causing tissue damage if use for cleaning and antisepsis is prolonged.
Elemental iodine (I2) is poisonous if taken orally in larger amounts; 2–3 grams of it is a lethal dose for an adult human.
Iodine vapor is very irritating to the eye
, to mucous membranes, and in the respiratory tract. Concentration of iodine in the air should not exceed 1 mg/m3 (eight-hour time-weighted average).
gland and disorders in functioning and growth of the organism as a whole. Iodides are similar in toxicity to bromide
s.
Excess iodine can be more cytotoxic
in the presence of selenium deficiency
.
Iodine supplementation in selenium-deficient populations is, in theory, problematic, partly for this reason.
can cause a rash. Some cases of reaction to Povidone-iodine (Betadine) have been documented to be a chemical burn. Eating iodine-containing foods can cause hives. Medical use of iodine (i.e. as a contrast agent, see above) can cause anaphylactic shock in highly iodine-sensitive patients. Some cases of sensitivity to iodine can be formally classified as iodine allergies. Iodine sensitivity is rare but has a considerable effect given the extremely widespread use of iodine-based contrast media.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with the symbol I and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
53. The name is pronounced icon , ˈ , or (British English) ˈ . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.
Iodine and its compounds are primarily used in nutrition
Nutrition
Nutrition is the provision, to cells and organisms, of the materials necessary to support life. Many common health problems can be prevented or alleviated with a healthy diet....
, and industrially in the production of acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
and certain polymers. Iodine's relatively high atomic number, low toxicity, and ease of attachment to organic compounds have made it a part of many X-ray contrast
Radiocontrast
Radiocontrast agents are a type of medical contrast medium used to improve the visibility of internal bodily structures in an X-ray based imaging techniques such as computed tomography or radiography...
materials in modern medicine. Iodine has only one stable isotope
Stable isotope
Stable isotopes are chemical isotopes that may or may not be radioactive, but if radioactive, have half-lives too long to be measured.Only 90 nuclides from the first 40 elements are energetically stable to any kind of decay save proton decay, in theory...
. A number of iodine radioisotopes are also used in medical applications.
Iodine is found on Earth mainly as the highly water-soluble iodide I-, which concentrates it in oceans and brine pools. Like the other halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s, free iodine occurs mainly as a diatomic
Diatomic
Diatomic molecules are molecules composed only of two atoms, of either the same or different chemical elements. The prefix di- means two in Greek. Common diatomic molecules are hydrogen , nitrogen , oxygen , and carbon monoxide . Seven elements exist in the diatomic state in the liquid and solid...
molecule I2, and then only momentarily after being oxidized from iodide by an oxidant like free oxygen. In the universe and on Earth, iodine's high atomic number makes it a relatively rare element
Abundance of the chemical elements
The abundance of a chemical element measures how relatively common the element is, or how much of the element is present in a given environment by comparison to all other elements...
, ranking 47th in abundance. However, its presence in ocean water has given it a role in biology. It is the heaviest essential element utilized widely by life in biological functions (only tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
, employed in enzymes by a few species of bacteria, is heavier). Iodine's rarity in many soils, due to initial low abundance as a crust-element, and also leaching of soluble iodide by rainwater, has led to many deficiency problems in land animals and inland human populations. Iodine deficiency
Iodine deficiency
Iodine is an essential trace element; the thyroid hormones thyroxine and triiodotyronine contain iodine. In areas where there is little iodine in the diet—typically remote inlandareas where no marine foods are eaten—iodine deficiency gives rise to...
affects about two billion people and is the leading preventable cause of intellectual disabilities.
Iodine is required by higher animals, which use it to synthesize thyroid hormones, which contain the element. Because of this function, radioisotopes of iodine are concentrated in the thyroid gland along with nonradioactive iodine. The radioisotope iodine-131
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
, which has a high fission product yield
Fission product yield
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.Yield can be broken down by:#Individual isotope...
, concentrates in the thyroid, and is one of the most carcinogenic of nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
products.
Characteristics
Iodine under standard conditions is a bluish-black solid. It can be seen apparently sublimating at standard temperatures into a violet-pink gas that has an irritating odor. This halogen forms compounds with many elements, but is less reactive than the other members of its Group VII (halogens) and has some metallic light reflectance.Elemental iodine dissolves easily in most organic solvents such as hexane
Hexane
Hexane is a hydrocarbon with the chemical formula C6H14; that is, an alkane with six carbon atoms.The term may refer to any of four other structural isomers with that formula, or to a mixture of them. In the IUPAC nomenclature, however, hexane is the unbranched isomer ; the other four structures...
or chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
owing to its lack of polarity, but is only slightly soluble in water. However, the solubility of elemental iodine in water can be increased by the addition of potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
. The molecular iodine reacts reversibly with the negative ion, generating the triiodide
Triiodide
In chemistry, triiodide can have several meanings. Triiodide primarily refers to the triiodide ion, I3−, a polyatomic anion composed of three iodine atoms. For some chemical compounds, triiodide indicates a salt of the named cation with the triiodide anion. Examples include sodium triiodide, ...
anion I3− in equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, which is soluble in water. This is also the formulation of some types of medicinal (antiseptic) iodine, although tincture of iodine
Tincture of iodine
Tincture of iodine is a disinfectant, usually 2–7% elemental iodine, along with potassium iodide or sodium iodide, dissolved in a mixture of ethanol and water. As in the case of Lugol's iodine, the role of iodide and water in the solution is to increase the solubility of the elemental iodine, by...
classically dissolves the element in aqueous ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
.
The colour of solutions of elemental iodine change depends on the polarity of the solvent. In non-polar solvents like hexane, solution are violet; in moderately polar dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
, the solution is dark crimson, and, in strongly polar solvents such as acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
or ethanol, it appears orange or brown. This effect is due to the formation of adducts.
Iodine melts at the relatively low temperature of 113.7 °C, although the liquid is often obscured by a dense violet vapor of gaseous iodine.
Occurrence

Solar System
The Solar System consists of the Sun and the astronomical objects gravitationally bound in orbit around it, all of which formed from the collapse of a giant molecular cloud approximately 4.6 billion years ago. The vast majority of the system's mass is in the Sun...
and Earth's crust (47th in abundance); however, iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
salts are often very soluble
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
in water. Iodine occurs in slightly greater concentrations in seawater
Seawater
Seawater is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% . This means that every kilogram of seawater has approximately of dissolved salts . The average density of seawater at the ocean surface is 1.025 g/ml...
than in rocks, 0.05 vs 0.04 ppm. Minerals containing iodine include caliche
Caliche (Mineral)
Caliche is a sedimentary rock, a hardened deposit of calcium carbonate. This calcium carbonate cements together other materials, including gravel, sand, clay, and silt. It is found in aridisol and mollisol soil orders...
, found in Chile
Chile
Chile ,officially the Republic of Chile , is a country in South America occupying a long, narrow coastal strip between the Andes mountains to the east and the Pacific Ocean to the west. It borders Peru to the north, Bolivia to the northeast, Argentina to the east, and the Drake Passage in the far...
. The brown algae
Algae
Algae are a large and diverse group of simple, typically autotrophic organisms, ranging from unicellular to multicellular forms, such as the giant kelps that grow to 65 meters in length. They are photosynthetic like plants, and "simple" because their tissues are not organized into the many...
Laminaria
Laminaria
Laminaria is a genus of 31 species of brown algae , all sharing the common name "kelp". This economically important genus is characterized by long, leathery laminae and relatively large size. Some species are referred to by the common name Devil's apron, due to their shape, or sea colander, due to...
and Fucus
Fucus
Fucus is a genus of brown algae found in the intertidal zones of rocky seashores almost throughout the world.-Description and life cycle:...
found in temperate zones of the Northern Hemisphere contain 0.028–0.454 dry weight percent of iodine. Aside from tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
, iodine is the heaviest element to be essential in living organisms, and iodine is the heaviest element thought to be needed by higher animals. About 19,000 tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s are produced annually from natural sources.
Organoiodine compound
Organoiodine compound
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature...
s are produced by marine life forms, the most notable being iodomethane (commonly called methyl iodide). The total iodomethane that is produced by the marine environment, by microbial activity in rice paddies and by the burning of biological material is estimated to be 214 kilotonnes/year. The volatile iodomethane is broken up in the atmosphere as part of a global iodine cycle.
Structure and bonding
Iodine normally exists as a diatomic molecule with a I-I bond length of 270 pm, one of the longest single bonds known. The I2 molecules tend to interact via the weak van der WaalsVan der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
force called the London Forces
London dispersion force
London dispersion forces is a type of force acting between atoms and molecules. They are part of the van der Waals forces...
, and this interaction is responsible for the higher melting point compared to more compact halogens, which are also diatomic. Since the atomic size of Iodine is larger, its melting point is higher. The solid crystallizes as orthorhombic crystals. The crystal motif in the Hermann–Mauguin notation is Cmca (No 64), Pearson symbol
Pearson symbol
The Pearson symbol, or Pearson notation, is used in crystallography as a means of describing a crystal structure, and was originated by W.B. Pearson. The symbol is made up of two letters followed by a number. For example:* Diamond structure, cF8...
oS8. The I-I bond is relatively weak, with a bond dissociation energy
Bond dissociation energy
In chemistry, bond-dissociation energy or D0, is one measure of the bond strength in a chemical bond. It is defined as the standard enthalpy change when a bond is cleaved by homolysis, with reactants and products of the homolysis reaction at 0 K...
of 36 kcal/mol. In fact, most bonds to iodine tend to be weaker than for the lighter halides. One consequence of this weak bonding is the relatively high tendency of I2 molecules to dissociate into atomic iodine.
Production
Of the several places in which iodine occurs in nature, only two sources are useful commercially: the calicheCaliche (Mineral)
Caliche is a sedimentary rock, a hardened deposit of calcium carbonate. This calcium carbonate cements together other materials, including gravel, sand, clay, and silt. It is found in aridisol and mollisol soil orders...
, found in Chile
Chile
Chile ,officially the Republic of Chile , is a country in South America occupying a long, narrow coastal strip between the Andes mountains to the east and the Pacific Ocean to the west. It borders Peru to the north, Bolivia to the northeast, Argentina to the east, and the Drake Passage in the far...
, and the iodine-containing brines of gas and oil fields, especially in Japan and the United States. The caliche, found in Chile, contains sodium nitrate
Sodium nitrate
Sodium nitrate is the chemical compound with the formula NaNO3. This salt, also known as Chile saltpeter or Peru saltpeter to distinguish it from ordinary saltpeter, potassium nitrate, is a white solid which is very soluble in water...
, which is the main product of the mining activities and small amounts of sodium iodate and sodium iodide. In the extraction of sodium nitrate, the sodium iodate and sodium iodide are extracted. The high concentration of iodine in the caliche and the extensive mining made Chile the largest producer of iodine in 2007.
Most other producers use natural occurring brine for the production of iodine. The Japanese Minami Kanto gas field
Minami Kanto gas field
The is a large gas field in Japan, west of Tokyo, in the Chiba prefecture.-Natural gas:The basin holds the most prolific natural gas reserves in Japan, with ultimate gas production of 375 billion cubic meters.-Brine:...
east of Tokyo
Tokyo
, ; officially , is one of the 47 prefectures of Japan. Tokyo is the capital of Japan, the center of the Greater Tokyo Area, and the largest metropolitan area of Japan. It is the seat of the Japanese government and the Imperial Palace, and the home of the Japanese Imperial Family...
and the American Anadarko Basin
Anadarko Basin
The Anadarko Basin is a geologic depositional and structural basin centered in the western part of the state of Oklahoma and the Texas Panhandle, and extending into western Kansas and southeast Colorado.-Geology:...
gas field in northwest Oklahoma
Oklahoma
Oklahoma is a state located in the South Central region of the United States of America. With an estimated 3,751,351 residents as of the 2010 census and a land area of 68,667 square miles , Oklahoma is the 28th most populous and 20th-largest state...
are the two largest sources for iodine from brine. The brine has a temperature of over 60°C owing to the depth of the source. The brine
Brine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
is first purified and acidified using sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, then the iodide present is oxidized to iodine with chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
. An iodine solution is produced, but is dilute and must be concentrated. Air is blown into the solution, causing the iodine to evaporate, then it is passed into an absorbing tower containing acid where sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
is added to reduce
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
the iodine. The hydrogen iodide
Hydrogen iodide
Hydrogen iodide is a diatomic molecule. Aqueous solutions of HI are known as iohydroic acid or hydroiodic acid, a strong acid. Gas and aqueous solution are interconvertible...
(HI) is reacted with chlorine to precipitate the iodine. After filtering and purification the iodine is packed.
- 2 HI + Cl2 → I2↑ + 2 HCl
- I2 + 2 H2O + SO2 → 2 HI + H2SO4
- 2 HI + Cl2 → I2↓ + 2 HCl
The production of iodine from seawater via electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
is not used owing to the sufficient abundance of iodine-rich brine. Another source of iodine was kelp
Kelp
Kelps are large seaweeds belonging to the brown algae in the order Laminariales. There are about 30 different genera....
, used in the 18th and 19th centuries, but it is no longer economically viable.

Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
with copper(II) sulfate, which gives copper(II) iodide initially. That decomposes spontaneously to copper(I) iodide
Copper(I) iodide
Copper iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding....
and iodine:
- Cu2+ + 2 I– → CuI2
- 2 CuI2 → 2 CuI + I2
There are also other methods of isolating this element in the laboratory, for example, the method used to isolate other halogens: Oxidation of the iodide in hydrogen iodide
Hydrogen iodide
Hydrogen iodide is a diatomic molecule. Aqueous solutions of HI are known as iohydroic acid or hydroiodic acid, a strong acid. Gas and aqueous solution are interconvertible...
(often made in situ with an iodide and sulfuric acid) by manganese dioxide (see below in Descriptive chemistry).
Isotopes and their applications
Of the 37 known (characterized) isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of iodine, only one, 127I, is stable.
The longest-lived radioisotope, 129I, has a half-life of 15.7 million years. This is long enough to make it a permanent fixture of the environment on human time scales, but far too short for it to exist as a primordial isotope today. Instead, iodine-129
Iodine-129
Iodine-129 is long-lived radioisotope of iodine which occurs naturally, but also is of special interest in the monitoring and effects of man-made nuclear fission decay products, where it serves as both tracer and potential radiological contaminant....
is an extinct radionuclide
Extinct radionuclide
An extinct radionuclide is one that scientists believe was formed by primordial processes, such as stellar nucleogenesis in the supernova that contributed radioisotopes to the early solar system, about 4.6 billion years ago...
, and its presence in the early solar system is inferred from the observation of an excess of its daughter xenon-129. This nuclide is also newly-made by cosmic rays and as a byproduct of human nuclear fission, which it is used to monitor as a very long-lived environmental contaminant.
The next-longest-lived radioisotope, iodine-125
Iodine-125
Iodine-125 is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat prostate cancer and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.Its half-life is around 59 days and it...
, has a half-life of 59 days. It is used as a convenient gamma-emitting tag for proteins in biological assays, and a few nuclear medicine
Nuclear medicine
In nuclear medicine procedures, elemental radionuclides are combined with other elements to form chemical compounds, or else combined with existing pharmaceutical compounds, to form radiopharmaceuticals. These radiopharmaceuticals, once administered to the patient, can localize to specific organs...
imaging tests where a longer half-life is required. It is also commonly used in brachytherapy
Brachytherapy
Brachytherapy , also known as internal radiotherapy, sealed source radiotherapy, curietherapy or endocurietherapy, is a form of radiotherapy where a radiation source is placed inside or next to the area requiring treatment...
implanted capsules, which kill tumors by local short-range gamma radiation (but where the isotope is never released into the body).
Iodine-123
Iodine-123
Iodine-123 is a radioactive isotope of iodine used in nuclear medicine imaging, including single photon emission computed tomography . The isotope's half-life is 13.22 hours; the decay by electron capture to tellurium-123 emits gamma radiation with predominant energies of 159 keV and 127 keV...
(half-life 13 hours) is the isotope of choice for nuclear medicine imaging of the thyroid gland, which naturally accumulates all iodine isotopes.
Iodine-131
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
(half-life 8 days) is a beta-emitting isotope, which is a common nuclear fission product. It is preferably administered to humans only in very high doses which destroy all tissues that accumulate it (usually the thyroid), which in turn prevents these tissues from developing cancer from a lower dose (paradoxically, a high dose of this isotope appears safer for the thryoid than a low dose). Like other radioiodines, I-131 accumulates in the thyroid gland, but unlike the others, in small amounts it is highly carcinogenic there, it seems, owing to the high local cell mutation due to damage from beta decay
Beta decay
In nuclear physics, beta decay is a type of radioactive decay in which a beta particle is emitted from an atom. There are two types of beta decay: beta minus and beta plus. In the case of beta decay that produces an electron emission, it is referred to as beta minus , while in the case of a...
. Because of this tendency of 131I to cause high damage to cells that accumulate it and other cells near them (0.6 to 2 mm away, the range of the beta rays), it is the only iodine radioisotope used as direct therapy, to kill tissues such as cancers that take up artificially iodinated molecules (example, the compound iobenguane
Iobenguane
Iobenguane, also known as metaiodobenzylguanidine or mIBG, or MIBG is a radiopharmaceutical, used in a scintigraphy method called MIBG scan...
, also known as MIBG). For the same reason, only the iodine isotope I-131 is used to treat Grave's disease and those types of thyroid cancers (sometimes in metastatic form) where the tissue that requires destruction, still functions to naturally accumulate iodide.
Nonradioactive ordinary potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
(iodine-127), in a number of convenient forms (tablets or solution) may be used to saturate the thyroid gland's ability to take up further iodine, and thus protect against accidental contamination from iodine-131 generated by nuclear fission
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is a nuclear reaction in which the nucleus of an atom splits into smaller parts , often producing free neutrons and photons , and releasing a tremendous amount of energy...
accidents, such as the Chernobyl disaster
Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the Chernobyl Nuclear Power Plant in Ukraine , which was under the direct jurisdiction of the central authorities in Moscow...
and more recently the Fukushima I nuclear accidents, as well as from contamination from this isotope in nuclear fallout
Nuclear fallout
Fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and shock wave have passed. It commonly refers to the radioactive dust and ash created when a nuclear weapon explodes...
from nuclear weapon
Nuclear weapon
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission or a combination of fission and fusion. Both reactions release vast quantities of energy from relatively small amounts of matter. The first fission bomb test released the same amount...
s.
History
Iodine was discovered by Bernard CourtoisBernard Courtois
Bernard Courtois, also spelled Barnard Courtois, was a French chemist born in Dijon, France.- Early life :...
in 1811. He was born to a manufacturer of saltpeter
Potassium nitrate
Potassium nitrate is a chemical compound with the formula KNO3. It is an ionic salt of potassium ions K+ and nitrate ions NO3−.It occurs as a mineral niter and is a natural solid source of nitrogen. Its common names include saltpetre , from medieval Latin sal petræ: "stone salt" or possibly "Salt...
(a vital part of gunpowder
Gunpowder
Gunpowder, also known since in the late 19th century as black powder, was the first chemical explosive and the only one known until the mid 1800s. It is a mixture of sulfur, charcoal, and potassium nitrate - with the sulfur and charcoal acting as fuels, while the saltpeter works as an oxidizer...
). At the time of the Napoleonic Wars
Napoleonic Wars
The Napoleonic Wars were a series of wars declared against Napoleon's French Empire by opposing coalitions that ran from 1803 to 1815. As a continuation of the wars sparked by the French Revolution of 1789, they revolutionised European armies and played out on an unprecedented scale, mainly due to...
, France
France
The French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
was at war and saltpeter was in great demand. Saltpeter produced from French niter
Niter
Niter or nitre is the mineral form of potassium nitrate, KNO3, also known as saltpeter or saltpetre . Historically, the term "niter" – cognate with "natrium", a Latin word for sodium – has been very vaguely defined, and it has been applied to a variety of other minerals and chemical compounds,...
beds required sodium carbonate
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
, which could be isolated from seaweed
Seaweed
Seaweed is a loose, colloquial term encompassing macroscopic, multicellular, benthic marine algae. The term includes some members of the red, brown and green algae...
collected on the coasts of Normandy
Normandy
Normandy is a geographical region corresponding to the former Duchy of Normandy. It is in France.The continental territory covers 30,627 km² and forms the preponderant part of Normandy and roughly 5% of the territory of France. It is divided for administrative purposes into two régions:...
and Brittany
Brittany
Brittany is a cultural and administrative region in the north-west of France. Previously a kingdom and then a duchy, Brittany was united to the Kingdom of France in 1532 as a province. Brittany has also been referred to as Less, Lesser or Little Britain...
. To isolate the sodium carbonate, seaweed was burned and the ash washed with water. The remaining waste was destroyed by adding sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
. Courtois once added excessive sulfuric acid and a cloud of purple vapor rose. He noted that the vapor crystallized on cold surfaces, making dark crystals. Courtois suspected that this was a new element but lacked funding to pursue it further.
Courtois gave samples to his friends, Charles Bernard Desormes
Charles Bernard Desormes
Charles Bernard Desormes was a French physicist and chemist. He determined the ratio of the specific heats of gases in 1819. He did this and almost all his scientific work in collaboration with his son-in-law Nicolas Clément...
(1777–1862) and Nicolas Clément (1779–1841), to continue research. He also gave some of the substance to chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac
- External links :* from the American Chemical Society* from the Encyclopædia Britannica, 10th Edition * , Paris...
(1778–1850), and to physicist
Physicist
A physicist is a scientist who studies or practices physics. Physicists study a wide range of physical phenomena in many branches of physics spanning all length scales: from sub-atomic particles of which all ordinary matter is made to the behavior of the material Universe as a whole...
André-Marie Ampère
André-Marie Ampère
André-Marie Ampère was a French physicist and mathematician who is generally regarded as one of the main discoverers of electromagnetism. The SI unit of measurement of electric current, the ampere, is named after him....
(1775–1836). On 29 November 1813, Dersormes and Clément made public Courtois's discovery. They described the substance to a meeting of the Imperial Institute of France. On December 6, Gay-Lussac announced that the new substance was either an element or a compound of oxygen. It was Gay-Lussac who suggested the name "iode", from the Greek word ιώδες (iodes) for violet (because of the color of iodine vapor). Ampère had given some of his sample to Humphry Davy
Humphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
(1778–1829). Davy did some experiments on the substance and noted its similarity to chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
. Davy sent a letter dated December 10 to the Royal Society of London stating that he had identified a new element. Arguments erupted between Davy and Gay-Lussac over who identified iodine first, but both scientists acknowledged Courtois as the first to isolate the element.
Catalysis
The major application of iodine is as a co-catalyst for the production of acetic acidAcetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
by the Monsanto
Monsanto process
The Monsanto process is an important method for the manufacture of acetic acid by catalytic carbonylation of methanol. This process operates at a pressure of 30–60 atm and a temperature of 150–200 °C and gives a selectivity greater than 99%. It was developed 1960 by German BASF and...
and Cativa process
Cativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc...
es. In these technologies, which support the world's demand for acetic acid, hydroiodic acid converts the methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
feedstock into methyl iodide, which undergoes carbonylation
Carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
. Hydrolysis of the resulting acetyl iodide regenerates hydroiodic acid and gives acetic acid.
Animal feed
The production of ethylenediammonium diiodide (EDDI) consumes a large fraction of available iodine. EDDI is provided to livestock as a nutritional supplement.Disinfectant and water treatment
Elemental iodine is used as a disinfectant in various forms. The iodine exists as the element, or as the water-soluble triiodideTriiodide
In chemistry, triiodide can have several meanings. Triiodide primarily refers to the triiodide ion, I3−, a polyatomic anion composed of three iodine atoms. For some chemical compounds, triiodide indicates a salt of the named cation with the triiodide anion. Examples include sodium triiodide, ...
anion I3- generated in situ by adding iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
to poorly water-soluble elemental iodine (the reverse chemical reaction makes some free elemental iodine available for antisepsis). In alternative fashion, iodine may come from iodophor
Iodophor
An Iodophor is a preparation containing iodine complexed with a solubilizing agent, such as a surfactant or povidone . The result is a water-soluble material that releases free iodine when in solution...
s, which contain iodine complexed with a solubilizing agent (iodide ion may be thought of loosely as the iodophor in triiodide water solutions). Examples of such preparations include:
- Tincture of iodineTincture of iodineTincture of iodine is a disinfectant, usually 2–7% elemental iodine, along with potassium iodide or sodium iodide, dissolved in a mixture of ethanol and water. As in the case of Lugol's iodine, the role of iodide and water in the solution is to increase the solubility of the elemental iodine, by...
: iodine in ethanol, or iodine and sodium iodideSodium iodideSodium iodide is a white, crystalline salt with chemical formula NaI used in radiation detection, treatment of iodine deficiency, and as a reactant in the Finkelstein reaction.-Uses:Sodium iodide is commonly used to treat and prevent iodine deficiency....
in a mixture of ethanol and water. - Lugol's iodineLugol's iodineLugol's iodine, also known as Lugol's solution, first made in 1829, is a solution of elemental iodine and potassium iodide in water, named after the French physician J.G.A. Lugol. Lugol's iodine solution is often used as an antiseptic and disinfectant, for emergency disinfection of drinking water,...
: iodine and iodide in water alone, forming mostly triiodide. Unlike tincture of iodine, Lugol's has a minimized amount of the free iodine (I2) component. - Povidone iodine (an iodophorIodophorAn Iodophor is a preparation containing iodine complexed with a solubilizing agent, such as a surfactant or povidone . The result is a water-soluble material that releases free iodine when in solution...
)
Health, medical, and radiological use
In most countries, table salt is iodized. Iodine is required for the essential thyroxin hormones produced by and concentrated in the thyroid gland.Potassium iodide has been used as an expectorant, although this use is increasingly uncommon. In medicine, potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
is used to treat acute thyrotoxicosis, usually as a saturated solution of potassium iodide (SSKI). It is also used to block uptake of iodine-131
Iodine-131
Iodine-131 , also called radioiodine , is an important radioisotope of iodine. It has a radioactive decay half-life of about eight days. Its uses are mostly medical and pharmaceutical...
in the thyroid gland (see isotopes section above), when this isotope is used as part of radiopharmaceuticals (such as iobenguane
Iobenguane
Iobenguane, also known as metaiodobenzylguanidine or mIBG, or MIBG is a radiopharmaceutical, used in a scintigraphy method called MIBG scan...
) that are not targetted to the thyroid or thyroid type tissues.
Iodine-131 (in the chemical form of iodide) is a component of nuclear fallout
Nuclear fallout
Fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and shock wave have passed. It commonly refers to the radioactive dust and ash created when a nuclear weapon explodes...
and a particularly dangerous one owing to the thyroid gland's propensity to concentrate ingested iodine, where it is kept for periods longer than this isotope's radiological half-life of eight days. For this reason, if people are expected to be exposed to a significant amount of environmental radioactive iodine (iodine-131 in fallout), they may be instructed to take non-radioactive potassium iodide tablets. The typical adult dose is one 130 mg tablet per 24 hours, supplying 100 mg (100,000 micrograms) iodine, as iodide ion. (Note: typical daily dose of iodine to maintain normal health is of order 100 micrograms; see "Dietary Intake" below.) By ingesting this large amount of non-radioactive iodine, radioactive iodine uptake by the thyroid gland is minimized. See the main article above for more on this topic.
Radiocontrast agent

Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
as X-ray radiocontrast
Radiocontrast
Radiocontrast agents are a type of medical contrast medium used to improve the visibility of internal bodily structures in an X-ray based imaging techniques such as computed tomography or radiography...
agents for intravenous injection. This is often in conjunction with advanced X-ray techniques such as angiography and CT scanning. At present, all water-soluble radiocontrast agents rely on iodine.
Other uses
Inorganic iodides find specialized uses. Hafnium, zirconium, titanium are purified by the van Arkel Process, which involves the reversible formation of the tetraiodides of these elements. Silver iodide is a major ingredient to traditional photographic film. Thousands of kilograms of silver iodide are consumed annually for cloud seedingCloud seeding
Cloud seeding, a form of intentional weather modification, is the attempt to change the amount or type of precipitation that falls from clouds, by dispersing substances into the air that serve as cloud condensation or ice nuclei, which alter the microphysical processes within the cloud...
.
The organoiodine compound erythrosine
Erythrosine
Erythrosine, also known as Red No. 3, is an organoiodine compound, specifically a derivative of fluorone. It is cherry-pink synthetic, primarily used for food coloring. It is the disodium salt of 2,4,5,7-tetraiodofluorescein...
is an important food coloring agent. Perfluoroalkyl iodides are precursors to important surfactants, such as perfluorooctanesulfonic acid.
Iodine chemistry
Iodine adopts a variety of oxidation states, commonly ranging from (formally) I7+ to I-, and including the intermediate states of I5+, I3+ and I+. Practically, only the 1- oxidation state is of significance, being the form found in iodide salts and organoiodine compoundOrganoiodine compound
Organoiodine compounds are organic compounds that contain one or more carbon–iodine bonds. They occur widely in organic chemistry, but are relatively rare in nature...
s.
Solubility
Being a nonpolar molecule, iodine is highly soluble in nonpolar organic solvents, including ethanolEthanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
(20.5 g/100 ml at 15 °C, 21.43 g/100 ml at 25 °C), diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
(20.6 g/100 ml at 17 °C, 25.20 g/100 ml at 25 °C), chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
, acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
, glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
, benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
(14.09 g/100 ml at 25 °C), carbon tetrachloride
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
(2.603 g/100 ml at 35 °C), and carbon disulfide
Carbon disulfide
Carbon disulfide is a colorless volatile liquid with the formula CS2. The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical non-polar solvent...
(16.47 g/100 ml at 25 °C). Elemental iodine is poorly soluble in water, with one gram dissolving in 3450 ml at 20 °C and 1280 ml at 50 °C. Aqueous and ethanol solutions are brown reflecting the role of these solvents as Lewis bases. Solutions in chloroform, carbon tetrachloride, and carbon disulfide are violet, the color of iodine vapor.
One of the most distinctive properties of iodine is the way that its solubility in water is enhanced by the presence of iodide ions. The dissolution of iodine in aqueous solutions containing iodide (e.g., from hydroiodic acid, potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
, etc.) results from the formation of the I3−
Triiodide
In chemistry, triiodide can have several meanings. Triiodide primarily refers to the triiodide ion, I3−, a polyatomic anion composed of three iodine atoms. For some chemical compounds, triiodide indicates a salt of the named cation with the triiodide anion. Examples include sodium triiodide, ...
ion. Dissolved bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
s also improve water solubility of iodine.
Redox reactions
In everyday life, iodides are slowly oxidized by atmospheric oxygen in the atmosphere to give free iodine. Evidence for this conversion is the yellow tint of certain aged samples of iodide salts and some organoiodine compounds. The oxidation of iodide to iodine in air is also responsible for the slow loss of iodide content in iodized salt if exposed to air. Some salts use iodate to prevent the loss of iodine.Iodine is easily oxidized and easily reduced. Most common is the interconversion of I- and I2. Molecular iodine can be prepared by oxidizing iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
s with chlorine:
- 2 I− + Cl2 → I2 + 2 Cl−
or with manganese dioxide in acid solution:
- 2 I− + 4 H+ + MnO2 → I2 + 2 H2O + Mn2+
Iodine is reduced to hydroiodic acid by hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
and hydrazine
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
:
- 8 I2 + 8 H2S → 16 HI + S8
- 2 I2 + N2H4 → 4 HI + N2
When dissolved in fuming sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
(65% oleum), iodine forms an intense blue solution. The blue color is due to cation, the result of iodine being oxidized by :
- 2 + 2 + → 2 + + 2
The cation is also formed in the oxidation of iodine by
Antimony pentafluoride
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a valuable Lewis acid and a component of the superacid fluoroantimonic acid, the strongest known acid...
or . The resulting or can be isolated as deep blue crystals. The solutions of these salts turn red when cooled below −60°C, owing to the formation of the cation:
- 2
Under slightly more alkaline conditions, disproportionates into and an iodine(III) compound. Excess iodine can then react with to form (green) and (black).
Oxides of iodine
The best-known oxides are the anions, IO3– and IO4–, but several other oxides are known, such as the strong oxidant iodine pentoxideIodine pentoxide
Iodine pentoxide is the chemical compound with the formula I2O5. This iodine oxide is the anhydride of iodic acid. It is produced by dehydrating iodic acid at 200 °C in a stream of dry air:- Structure :...
.
By contrast with chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, the formation of the hypohalite ion (IO–) in neutral aqueous solutions of iodine is negligible.
- I2 + H2O H+ + I− + HIO (K = 2.0×10−13) In basic solutions (such as aqueous sodium hydroxide), iodine converts in a two stage reaction to iodideIodideAn iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
and iodateIodateAn iodate is a conjugate base of iodic acid. In the iodate anion, iodine is bonded to three oxygen atoms and the molecular formula is IO3−. The molecular geometry of iodate is trigonal pyramidal....
:I2 + 2 OH− → I− + IO− + H2O (K = 30) 3 IO− → 2 I− + IO3− (K = 1020)
Organic derivatives of hypoiodate (2-Iodoxybenzoic acid
2-Iodoxybenzoic acid
IBX acid or 2-iodoxybenzoic acid is an organic compound used in organic synthesis as an oxidizing agent. This Periodinane is especially suited to oxidize alcohols to aldehydes. The IBX acid is prepared from 2-iodobenzoic acid, potassium bromate and sulfuric acid...
, and Dess-Martin periodinane
Dess-Martin periodinane
Dess–Martin periodinane is a chemical reagent used to oxidize primary alcohols to aldehydes and secondary alcohols to ketones. This periodinane has several advantages over chromium- and DMSO-based oxidants that include milder conditions , shorter reaction times, higher yields, simplified workups,...
) are used in organic chemistry.
Iodic acid
Iodic acid
Iodic acid, HIO3, can be obtained as a white solid. It dissolves in water very well, but it also exists in the pure state, as opposed to chloric acid or bromic acid. Iodic acid contains iodine in the oxidation state +5 and it is one of the most stable oxo-acids of the halogens in its pure state....
(HIO3), periodic acid
Periodic acid
Periodic acid, or iodic acid is an oxoacid of iodine having chemical formula HIO4 or H5IO6.In dilute aqueous solution, periodic acid exists as discrete hydronium and metaperiodate ions. When more concentrated, orthoperiodic acid, H5IO6, is formed; this dissociates into hydronium and...
(HIO4) and their salts are strong oxidizers and are of some use in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. Iodine is oxidized to iodate
Iodate
An iodate is a conjugate base of iodic acid. In the iodate anion, iodine is bonded to three oxygen atoms and the molecular formula is IO3−. The molecular geometry of iodate is trigonal pyramidal....
by nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
as well as by chlorate
Chlorate
The chlorate anion has the formula ClO. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a roman numeral in parentheses, e.g...
s:
- I2 + 10 HNO3 → 2 HIO3 + 10 NO2 + 4 H2O
- I2 + 2 ClO3− → 2 IO3− + Cl2
Inorganic iodine compounds
Iodine forms compounds with all the elements except for the noble gases. From the perspective of commercial applications, an important compound is hydroiodic acid, used as a co-catalyst in the Cativa processCativa process
The Cativa process is a method for the production of acetic acid by the carbonylation of methanol. The technology, which is similar to the Monsanto process, was developed by BP Chemicals and is under license by BP Plc...
for the production of acetic acid. Titanium and aluminium iodides are used in the production of butadiene, a precursor to rubber tires.
Alkali metal salts are common colourless solids that are highly soluble in water. Potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
is a convenient source of the iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
anion; it is easier to handle than sodium iodide
Sodium iodide
Sodium iodide is a white, crystalline salt with chemical formula NaI used in radiation detection, treatment of iodine deficiency, and as a reactant in the Finkelstein reaction.-Uses:Sodium iodide is commonly used to treat and prevent iodine deficiency....
because it is not hygroscopic. Both salts are mainly used in the production of iodized salt. Sodium iodide is especially useful in the Finkelstein reaction
Finkelstein reaction
The Finkelstein reaction, named for the German chemist Hans Finkelstein , is an SN2 reaction that involves the exchange of one halogen atom for another...
, because it is soluble in acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
, whereas potassium iodide is less so. In this reaction, an alkyl chloride is converted to an alkyl iodide. This relies on the insolubility of sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
in acetone to drive the reaction:
- R-Cl (acetone) + NaI (acetone) → R-I (acetone) + NaCl (s)
Despite having the lowest electronegativity of the common halogens, iodine reacts violently with some metals, such as aluminium:
- 3 I2 + 2 Al → 2 AlI3
This reaction produces 314 kJ per mole of aluminum, comparable to thermite's 425 kJ. Yet the reaction initiates spontaneously, and if unconfined, causes a cloud of gaseous iodine due to the high temperature.
Interhalogen compounds
Interhalogen compounds are well known; examples include iodine monochlorideIodine monochloride
Iodine monochloride is an interhalogen compound with the formula ICl. It is a red-brown compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, ICl is highly polar and behaves as a source of I+....
and trichloride
Iodine trichloride
Iodine trichloride is an interhalogen compound of iodine and chlorine. It is bright yellow and in the solid state is present as a planar dimer I2Cl6, Cl2I2ICl2, with two bridging Cl atoms....
; iodine pentafluoride
Iodine pentafluoride
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is a fluoride of iodine. It is a colourless or yellow liquid with a density of 3.250 g cm−3. It was first synthesized by Henri Moissan in 1891 by burning solid iodine in fluorine gas...
and heptafluoride
Iodine heptafluoride
Iodine heptafluoride, also known as iodine fluoride or even iodine fluoride, is an interhalogen compound with chemical formula IF7. It has an unusual pentagonal bipyramidal structure, as predicted by VSEPR theory...
.
Organic compounds
Many organoiodine compounds exist; the simplest is iodomethaneIodomethane
Methyl iodide, also called iodomethane, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally...
, approved as a soil fumigant. Iodinated organic compounds are used as synthetic reagents.
Organic synthesis
Organoiodine compounds can be made in many ways. For example, methyl iodide can be prepared from methanolMethanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, red phosphorus, and iodine. The iodinating reagent is phosphorus triiodide
Phosphorus triiodide
Phosphorus triiodide is an unstable red solid which reacts violently with water. It is a common misconception that PI3 is too unstable to be stored; it is, in fact, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides. It is also a powerful...
that is formed in situ:
- 3 CH3OH + PI3 → 3 CH3I + Phosphorous acid
The iodoform test uses an alkaline solution of iodine to react with methyl ketones to give the labile triiodomethide leaving group, forming iodoform, which precipitates.
Aryl and alkyl iodides both form Grignard reagents. Iodine is sometimes used to activate magnesium when preparing Grignard reagents. Alkyl iodides such as iodomethane are good alkylating agents. Some drawbacks to use of organoiodine compounds in chemical synthesis are:
- iodine compounds tend to be more expensive than the corresponding bromides and chlorides, in that order
- iodides tend to be much stronger alkylating agents, and so are more toxic (e.g., methyl iodide is very toxic (T+).
- low-molecular-weight iodides tend to have a much higher equivalent weight, compared to other alkylating agents (e.g., methyl iodide versus dimethyl carbonate), owing to the atomic mass of iodine.
Analytical chemistry and bioanalysis

Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
and glycogen
Glycogen
Glycogen is a molecule that serves as the secondary long-term energy storage in animal and fungal cells, with the primary energy stores being held in adipose tissue...
. Several analytical methods rely on this property:
- IodometryIodometryIodometry, also known as iodometric titration, is a method of volumetric chemical analysis, a redox titration where the appearance or disappearance of elementary iodine indicates the end point....
. The concentration of an oxidant can be determined by adding it to an excess of iodide, to destroy elemental iodine/triiodide as a result of oxidation by the oxidant. A starch indicatorStarch indicatorStarch is often used in chemistry as an indicator for redox titrations where triiodide is present. Starch forms a very dark blue-black complex with triiodide which can be made by mixing iodine with iodide . However, the complex is not formed if only iodine or only iodide is present...
is then used as the indicatorRedox indicatorA redox indicator is an indicator that undergoes a definite color change at a specific electrode potential....
close to the end-point, in order to increase the visual contrast (dark blue becomes colorless, instead of the yellow of dilute triiodide becoming colorless). - An Iodine testIodine testThe Iodine test is used to test for the presence of starch. Iodine solution — iodine dissolved in an aqueous solution of potassium iodide — reacts with the starch producing a purple black color. The colour can be detected visually with concentrations of iodine as low as 0.00002M at 20°C...
may be used to test a sample substance for the presence of starch. The Iodine clock reactionIodine clock reactionThe iodine clock reaction is a classical chemical clock demonstration experiment to display chemical kinetics in action; it was discovered by Hans Heinrich Landolt in 1886. Two colorless solutions are mixed and at first there is no visible reaction...
is an extension of the techniques in iodometry. - Iodine solutions are used in counterfeit banknote detection pens; the premise being that counterfeit banknotes made using commercially available paper contain starch.
- Starch-iodide paper are used to test for the presence of oxidants such as peroxides. The oxidants convert iodide to iodine, which shows up as blue. A solution of starch and iodide can perform the same function.
- During colposcopyColposcopyColposcopy is a medical diagnostic procedure to examine an illuminated, magnified view of the cervix and the tissues of the vagina and vulva. Many premalignant lesions and malignant lesions in these areas have discernible characteristics which can be detected through the examination...
, Lugol's iodine is applied to the vaginaVaginaThe vagina is a fibromuscular tubular tract leading from the uterus to the exterior of the body in female placental mammals and marsupials, or to the cloaca in female birds, monotremes, and some reptiles. Female insects and other invertebrates also have a vagina, which is the terminal part of the...
and cervixCervixThe cervix is the lower, narrow portion of the uterus where it joins with the top end of the vagina. It is cylindrical or conical in shape and protrudes through the upper anterior vaginal wall...
. Normal vaginal tissue stains brown owing to its high glycogen content (a color-reaction similar to that with starch), while abnormal tissue suspicious for cancer does not stain, and thus appears pale compared to the surrounding tissue. BiopsyBiopsyA biopsy is a medical test involving sampling of cells or tissues for examination. It is the medical removal of tissue from a living subject to determine the presence or extent of a disease. The tissue is generally examined under a microscope by a pathologist, and can also be analyzed chemically...
of suspicious tissue can then be performed. This is called a Schiller's TestSchiller's TestSchiller's test or Schiller's Iodine test is a medical test in which iodine solution is applied to the cervix in order to diagnose cervical cancer.- Procedure :...
.
Clandestine synthetic chemical use
In the United States, the Drug Enforcement Agency (DEA) regards iodine and compounds containing iodine (ionic iodides, iodoform, ethyl iodide, and so on) as reagents useful for the clandestine manufacture of methamphetamineMethamphetamine
Methamphetamine is a psychostimulant of the phenethylamine and amphetamine class of psychoactive drugs...
.
Biological role
Iodine is an essential trace elementTrace element
In analytical chemistry, a trace element is an element in a sample that has an average concentration of less than 100 parts per million measured in atomic count, or less than 100 micrograms per gram....
for life, the heaviest element commonly needed by living organisms. Only tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
, a component of a few bacterial enzymes, has a higher atomic number and atomic weight.
Iodine's main role in animal biology is as a constituent of the thyroid
Thyroid
The thyroid gland or simply, the thyroid , in vertebrate anatomy, is one of the largest endocrine glands. The thyroid gland is found in the neck, below the thyroid cartilage...
hormone
Hormone
A hormone is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. In essence, it is a chemical messenger that transports a signal from one...
s thyroxine
Thyroxine
Thyroxine, or 3,5,3',5'-tetraiodothyronine , a form of thyroid hormones, is the major hormone secreted by the follicular cells of the thyroid gland.-Synthesis and regulation:...
(T4) and triiodothyronine
Triiodothyronine
Triiodothyronine, C15H12I3NO4, also known as T3, is a thyroid hormone. It affects almost every physiological process in the body, including growth and development, metabolism, body temperature, and heart rate....
(T3). These are made from addition condensation products of the amino acid tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
, and are stored prior to release in an iodine-containing protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
called thyroglobulin
Thyroglobulin
Thyroglobulin is a 660 kDa, dimeric protein produced by and used entirely within the thyroid gland. In earlier literature, Tg was referred to as colloid....
. T4 and T3 contain four and three atoms of iodine per molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
, respectively. The thyroid gland actively absorbs iodide from the blood
Blood
Blood is a specialized bodily fluid in animals that delivers necessary substances such as nutrients and oxygen to the cells and transports metabolic waste products away from those same cells....
to make and release these hormones into the blood, actions that are regulated by a second hormone TSH
Thyroid-stimulating hormone
Thyrotrophin-stimulating hormone is a peptide hormone synthesized and secreted by thyrotrope cells in the anterior pituitary gland, which regulates the endocrine function of the thyroid gland.- Physiology :...
from the pituitary. Thyroid hormones are phylogenetically very old molecules that are synthesized by most multicellular organisms, and that even have some effect on unicellular organisms.
Thyroid hormones play a basic role in biology, acting on gene transcription to regulate the basal metabolic rate
Basal metabolic rate
Basal Metabolic Rate , and the closely related resting metabolic rate , is the amount of daily energy expended by humans and other animals at rest. Rest is defined as existing in a neutrally temperate environment while in the post-absorptive state...
. The total deficiency of thyroid hormones can reduce basal metabolic rate up to 50%, while in excessive production of thyroid hormones the basal metabolic rate can be increased by 100%. T4 acts largely as a precursor to T3, which is (with minor exceptions) the biologically active hormone.
Iodine has a nutritional relationship with selenium
Selenium
Selenium is a chemical element with atomic number 34, chemical symbol Se, and an atomic mass of 78.96. It is a nonmetal, whose properties are intermediate between those of adjacent chalcogen elements sulfur and tellurium...
. A family of selenium-dependent enzymes called deiodinase
Deiodinase
Iodothyronine deiodinases are a subfamily of deiodinase enzymes important in the activation and deactivation of thyroid hormones. Thyroxine , the precursor of 3,5,3’-triiodothyronine is transformed into T3 by deiodinase activity. T3, through binding a nuclear thyroid hormone receptor,...
s converts T4 to T3 (the active hormone) by removing an iodine atom from the outer tyrosine ring. These enzymes also convert T4 to reverse T3 (rT3) by removing an inner ring iodine atom, and convert T3 to 3,3'-diiodothyronine
3,3'-Diiodothyronine
3,3'-Diiodothyronine is a metabolite of thyroid hormone.It is formed from the breakdown of triiodothyronine. It is an allosteric regulator of the Cytochrome C Oxidase, the complex IV of the electron transport chain. It increases its activity by preventing the interaction of ATP as an allosteric...
(T2) also by removing an inner ring atom. Both of the latter are inactivated hormones that are ready for disposal and have, in essence, no biological effects. A family of non-selenium-dependent enzymes then further deiodinates the products of these reactions.
Iodine accounts for 65% of the molecular weight of T4 and 59% of the T3. Fifteen to 20 mg of iodine is concentrated in thyroid tissue and hormones, but 70% of the body's iodine is distributed in other tissues, including mammary glands, eyes, gastric mucosa, the cervix, and salivary glands. In the cells of these tissues, iodide enters directly by sodium-iodide symporter
Sodium-iodide symporter
The sodium/iodide symporter , also known as solute carrier family 5, member 5 is a protein that in humans is encoded by the SLC5A5 gene. The sodium/iodide symporter is a transmembrane glycoprotein with a molecular weight of 87 kDa and 13 transmembrane domains, which transports two sodium cations ...
(NIS). Its role in mammary tissue is related to fetal and neonatal development, but its role in the other tissues is unknown.
Dietary intake
The daily Dietary Reference IntakeDietary Reference Intake
The Dietary Reference Intake is a system of nutrition recommendations from the Institute of Medicine of the U.S. National Academy of Sciences. The DRI system is used by both the United States and Canada and is intended for the general public and health professionals...
recommended by the United States Institute of Medicine
Institute of Medicine
The Institute of Medicine is a not-for-profit, non-governmental American organization founded in 1970, under the congressional charter of the National Academy of Sciences...
is between 110 and 130 µg
Microgram
In the metric system, a microgram is a unit of mass equal to one millionth of a gram , or 1/1000 of a milligram. It is one of the smallest units of mass commonly used...
for infants up to 12 months, 90 µg for children up to eight years, 130 µg for children up to 13 years, 150 µg for adults, 220 µg for pregnant women and 290 µg for lactating mothers
Lactation
Lactation describes the secretion of milk from the mammary glands and the period of time that a mother lactates to feed her young. The process occurs in all female mammals, however it predates mammals. In humans the process of feeding milk is called breastfeeding or nursing...
. The Tolerable Upper Intake Level (UL) for adults is 1,100 μg/day (1.1 mg/day). The tolerable upper limit was assessed by analyzing the effect of supplementation on thyroid-stimulating hormone
Thyroid-stimulating hormone
Thyrotrophin-stimulating hormone is a peptide hormone synthesized and secreted by thyrotrope cells in the anterior pituitary gland, which regulates the endocrine function of the thyroid gland.- Physiology :...
.
The thyroid gland needs no more than 70 micrograms /day to synthesize the requisite daily amounts of T4 and T3. The higher recommended daily allowance levels of iodine seem necessary for optimal function of a number of body systems, including lactating breast, gastric mucosa, salivary glands, oral mucosa, thymus, epidermis, choroid plexus, etc. The high iodide-concentration of thymus tissue in particular suggests an anatomical rationale for this role of iodine in the immune system. The trophic, antioxidant and apoptosis-inductor actions and the presumed anti-tumour activity of iodides has been suggested to also be important for prevention of oral and salivary glands diseases.
Natural sources of iodine include sea life, such as kelp
Kelp
Kelps are large seaweeds belonging to the brown algae in the order Laminariales. There are about 30 different genera....
and certain seafood, as well as plants grown on iodine-rich soil. Iodized salt is fortified with iodine.
As of 2000, the median intake of iodine from food in the United States was 240 to 300 μg/day for men and 190 to 210 μg/day for women. In Japan, consumption is much higher, owing to the frequent consumption of seaweed or kombu
Kombu
Kombu or konbu , also called dashima or haidai , is edible kelp from the family Laminariaceae widely eaten in East Asia....
kelp.
After iodine fortification programs (e.g., iodized salt) have been implemented, some cases of iodine-induced hyperthyroidism
Hyperthyroidism
Hyperthyroidism is the term for overactive tissue within the thyroid gland causing an overproduction of thyroid hormones . Hyperthyroidism is thus a cause of thyrotoxicosis, the clinical condition of increased thyroid hormones in the blood. Hyperthyroidism and thyrotoxicosis are not synonymous...
have been observed (so called Jod-Basedow phenomenon). The condition seems to occur mainly in people over forty, and the risk appears higher when iodine deficiency is severe and the initial rise in iodine intake is high.
Deficiency
In areas where there is little iodine in the diet, typically remote inland areas and semi-arid equatorial climates where no marine foods are eaten, iodine deficiencyIodine deficiency
Iodine is an essential trace element; the thyroid hormones thyroxine and triiodotyronine contain iodine. In areas where there is little iodine in the diet—typically remote inlandareas where no marine foods are eaten—iodine deficiency gives rise to...
gives rise to hypothyroidism, symptoms of which are extreme fatigue, goitre
Goitre
A goitre or goiter , is a swelling in the thyroid gland, which can lead to a swelling of the neck or larynx...
, mental slowing, depression, weight gain, and low basal body temperatures. Iodine deficiency is the leading cause of preventable mental retardation
Mental retardation
Mental retardation is a generalized disorder appearing before adulthood, characterized by significantly impaired cognitive functioning and deficits in two or more adaptive behaviors...
, a result that occurs primarily when babies or small children are rendered hypothyroidic by a lack of the element. The addition of iodine to table salt has largely eliminated this problem in the wealthier nations, but, as of March 2006, iodine deficiency remained a serious public health problem in the developing world. Iodine deficiency is also a problem in certain areas of Europe.
Other possible health effects being investigated as being related to deficiency include:
- Breast cancer. The breast strongly and actively concentrates iodine into breast-milk for the benefit of the developing infant, and may develop a goiter-like hyperplasia, sometimes manifesting as fibrocystic breast disease, when iodine level are low. Studies indicate that iodine deficiency, either dietary or pharmacologic, can lead to breast atypiaAtypiaAtypia is a clinical term for abnormality in a cell. The term is medical jargon for an atypical cell. Atypia: Etymology: Gk, a + typos, without type; a condition of being irregular or nonstandard....
and increased incidence of malignancy in animal models, while iodine treatment can reverse dysplasiaDysplasiaDysplasia , is a term used in pathology to refer to an abnormality of development. This generally consists of an expansion of immature cells, with a corresponding decrease in the number and location of mature cells. Dysplasia is often indicative of an early neoplastic process...
. The role of iodide in breast dysplasia and development of breast cancer is an area of active research.
- Stomach cancer. Some researchers have found an epidemiologic correlation between iodine deficiency, iodine-deficient goitre and gastric cancer. A decrease of the incidence of death rate from stomach cancer after implementation of the effective iodine-prophylaxis has been reported also.
Precautions and toxicity of elemental iodine
Elemental iodine is an oxidizing irritant and direct contact with skin can cause lesions, so iodine crystals should be handled with care. Solutions with high elemental iodine concentration such as tincture of iodineTincture of iodine
Tincture of iodine is a disinfectant, usually 2–7% elemental iodine, along with potassium iodide or sodium iodide, dissolved in a mixture of ethanol and water. As in the case of Lugol's iodine, the role of iodide and water in the solution is to increase the solubility of the elemental iodine, by...
and Lugol's solution are capable of causing tissue damage if use for cleaning and antisepsis is prolonged.
Elemental iodine (I2) is poisonous if taken orally in larger amounts; 2–3 grams of it is a lethal dose for an adult human.
Iodine vapor is very irritating to the eye
Human eye
The human eye is an organ which reacts to light for several purposes. As a conscious sense organ, the eye allows vision. Rod and cone cells in the retina allow conscious light perception and vision including color differentiation and the perception of depth...
, to mucous membranes, and in the respiratory tract. Concentration of iodine in the air should not exceed 1 mg/m3 (eight-hour time-weighted average).
Toxicity of iodide ion
Excess iodine has symptoms similar to those of iodine deficiency. Commonly encountered symptoms are abnormal growth of the thyroidThyroid
The thyroid gland or simply, the thyroid , in vertebrate anatomy, is one of the largest endocrine glands. The thyroid gland is found in the neck, below the thyroid cartilage...
gland and disorders in functioning and growth of the organism as a whole. Iodides are similar in toxicity to bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
s.
Excess iodine can be more cytotoxic
Cytotoxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are a chemical substance, an immune cell or some types of venom .-Cell physiology:...
in the presence of selenium deficiency
Selenium deficiency
Selenium deficiency is relatively rare in healthy well-nourished individuals. Few cases have been reported.-Causes:It can occur in patients with severely compromised intestinal function, those undergoing total parenteral nutrition, those who have had gastrointestinal bypass surgery, and also on...
.
Iodine supplementation in selenium-deficient populations is, in theory, problematic, partly for this reason.
Iodine sensitivity
Some people develop a sensitivity to iodine. Application of tincture of iodineTincture of iodine
Tincture of iodine is a disinfectant, usually 2–7% elemental iodine, along with potassium iodide or sodium iodide, dissolved in a mixture of ethanol and water. As in the case of Lugol's iodine, the role of iodide and water in the solution is to increase the solubility of the elemental iodine, by...
can cause a rash. Some cases of reaction to Povidone-iodine (Betadine) have been documented to be a chemical burn. Eating iodine-containing foods can cause hives. Medical use of iodine (i.e. as a contrast agent, see above) can cause anaphylactic shock in highly iodine-sensitive patients. Some cases of sensitivity to iodine can be formally classified as iodine allergies. Iodine sensitivity is rare but has a considerable effect given the extremely widespread use of iodine-based contrast media.
See also
- Iodide as an antioxidant
- Chemical oxygen iodine laserChemical oxygen iodine laserChemical oxygen iodine laser, or COIL, is an infrared chemical laser. As the beam is infrared, it cannot be seen with the naked eye. It is capable of output power scaling up to megawatts in continuous mode...
- Nutrition facts label
- Starch indicatorStarch indicatorStarch is often used in chemistry as an indicator for redox titrations where triiodide is present. Starch forms a very dark blue-black complex with triiodide which can be made by mixing iodine with iodide . However, the complex is not formed if only iodine or only iodide is present...
- InadineInadineInadine is a brand of non-adherent surgical dressing containing the disinfectant povidone-iodine . It is produced by Johnson & Johnson. This dressing is typically used on open wounds that may become infected, before or after surgery or even used on existing infected wounds...
External links
- "Micronutrient Research for Optimum Health", Linus Pauling Institute, OSU Oregon State University
- ATSDR – CSEM: Radiation Exposure from Iodine 131 U.S. Department of Health and Human Services (public domain)
- ChemicalElements.com – Iodine
- who.int, WHO Global Database on Iodine Deficiency
- Oxidizing Agents > Iodine

