
Organoiodine compound
Encyclopedia
Organoiodine compounds are organic compounds that contain one or more carbon
–iodine
bond
s. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine
hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
The C–I bond is the weakest of the carbon–halogen
bonds. These bond strengths correlate with the electronegativity
of the halogen, decreasing in the order F > Cl > Br > I. This periodic
order also follows the atomic radius
of halogens and the length of the carbon-halogen bond. For example, in the molecules represented by CH3X, where X is a halide, the carbon-X bonds have strengths, or bond dissociation energies, of 115, 83.7, 72.1, and 57.6 kcal/mol for X = fluoride, chloride, bromide, and iodide, respectively. Of the halides, iodide usually is the best leaving group
. Because of the weakness of the C-I bond, samples of organoiodine compounds are often yellow due to an impurity of I2.
A noteworthy aspect of organoiodine compounds is their high density, which arises from the high atomic weight of iodine. For example, one millilitre of methylene iodide weighs 3.325 g.
, because of the easy formation and cleavage of the C–I bond. Industrially significant organoiodine compounds, often used as disinfectants or pesticides, are iodoform
(CHI3), methylene iodide (CH2I2), and methyl iodide (CH3I). Although methyl iodide is not an industrially important product, it is an important intermediate, being a transiently generated intermediate in the industrial production of acetic acid
and acetic anhydride
.
Polyiodoorganic compounds are sometimes employed as X-ray contrast agents, in fluoroscopy
, a type of medical imaging
. This application exploits the X-ray absorbing ability of the heavy iodine nucleus. A variety of agents are available commercially, many are derivatives of 1,3,5-triiodobenzene
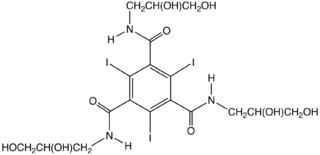
and contain about 50% by weight iodine. For most applications, the agent must be highly soluble in water and, of course, non-toxic and readily excreted. A representative reagent is Ioversol
(Figure to right), which has water-solubilizing diol
substituents. Typical applications include urography and angiography.
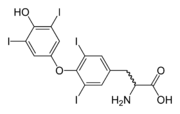
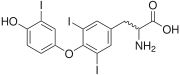
s thyroxine
("T4") and triiodothyronine
("T3"). Marine natural products are rich sources of organoiodine compounds, like the recently discovered plakohypaphorine
s from the sponge Plakortis simplex
.
 The sum of iodomethane
The sum of iodomethane
produced by the marine environment, microbial activitiy in rice paddies, and the burning of biological material is estimated to be 214 kilotonnes per year. The volatile iodomethane is broken up by oxidation reactions in the atmosphere and a global iodine cycle is established.
Moreover, iodine can add to double bonds of docosahexaenoic acid
and arachidonic acid
of cellular membranes, making iodolipids which seem to have an apoptic action and are less reactive to free oxygen radicals.
The iodide anion is a good nucleophile and will displace chloride, tosylate, bromide and other leaving groups, as in the Finkelstein reaction
. Aromatic iodides may be prepared via a diazonium salt in the Sandmeyer reaction
.
Because of its low ionization energy, iodine is readily converted to reagents that deliver the equivalent of "I+". A representative electrophilic iodination reagent is iodine monochloride
.
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
–iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine
Thyroxine
Thyroxine, or 3,5,3',5'-tetraiodothyronine , a form of thyroid hormones, is the major hormone secreted by the follicular cells of the thyroid gland.-Synthesis and regulation:...
hormones are organoiodine compounds that are required for health and the reason for government-mandated iodization of salt.
Structure, bonding, general properties
Almost all organoiodine compounds feature iodide connected to one carbon center. These are usually classified as derivatives of I-. Some organoiodine compounds feature iodine in higher oxidation states.The C–I bond is the weakest of the carbon–halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
bonds. These bond strengths correlate with the electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
of the halogen, decreasing in the order F > Cl > Br > I. This periodic
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
order also follows the atomic radius
Atomic radius
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons...
of halogens and the length of the carbon-halogen bond. For example, in the molecules represented by CH3X, where X is a halide, the carbon-X bonds have strengths, or bond dissociation energies, of 115, 83.7, 72.1, and 57.6 kcal/mol for X = fluoride, chloride, bromide, and iodide, respectively. Of the halides, iodide usually is the best leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
. Because of the weakness of the C-I bond, samples of organoiodine compounds are often yellow due to an impurity of I2.
A noteworthy aspect of organoiodine compounds is their high density, which arises from the high atomic weight of iodine. For example, one millilitre of methylene iodide weighs 3.325 g.
Industrial applications
Few organoiodine compounds are important industrially, at least in terms of large scale production. Iodide containing intermediates are common in organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, because of the easy formation and cleavage of the C–I bond. Industrially significant organoiodine compounds, often used as disinfectants or pesticides, are iodoform
Iodoform
Iodoform is the organoiodine compound with the formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating odor and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant...
(CHI3), methylene iodide (CH2I2), and methyl iodide (CH3I). Although methyl iodide is not an industrially important product, it is an important intermediate, being a transiently generated intermediate in the industrial production of acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
and acetic anhydride
Acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula 2O. Commonly abbreviated Ac2O, it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis...
.
Polyiodoorganic compounds are sometimes employed as X-ray contrast agents, in fluoroscopy
Fluoroscopy
Fluoroscopy is an imaging technique commonly used by physicians to obtain real-time moving images of the internal structures of a patient through the use of a fluoroscope. In its simplest form, a fluoroscope consists of an X-ray source and fluorescent screen between which a patient is placed...
, a type of medical imaging
Medical imaging
Medical imaging is the technique and process used to create images of the human body for clinical purposes or medical science...
. This application exploits the X-ray absorbing ability of the heavy iodine nucleus. A variety of agents are available commercially, many are derivatives of 1,3,5-triiodobenzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
and contain about 50% by weight iodine. For most applications, the agent must be highly soluble in water and, of course, non-toxic and readily excreted. A representative reagent is Ioversol
Ioversol
Ioversol is an organoiodine compound that used as a contrast medium. It features both a high iodine content, as well as several hydrophilic groups....
(Figure to right), which has water-solubilizing diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
substituents. Typical applications include urography and angiography.

Biological role
In terms of human health, the most important organoiodine compounds are the two thyroid hormoneThyroid hormone
The thyroid hormones, thyroxine and triiodothyronine , are tyrosine-based hormones produced by the thyroid gland primarily responsible for regulation of metabolism. An important component in the synthesis of thyroid hormones is iodine. The major form of thyroid hormone in the blood is thyroxine ,...
s thyroxine
Thyroxine
Thyroxine, or 3,5,3',5'-tetraiodothyronine , a form of thyroid hormones, is the major hormone secreted by the follicular cells of the thyroid gland.-Synthesis and regulation:...
("T4") and triiodothyronine
Triiodothyronine
Triiodothyronine, C15H12I3NO4, also known as T3, is a thyroid hormone. It affects almost every physiological process in the body, including growth and development, metabolism, body temperature, and heart rate....
("T3"). Marine natural products are rich sources of organoiodine compounds, like the recently discovered plakohypaphorine
Plakohypaphorine
Plakohypaphorines are iodine-containing indole alkaloids named for their similarity to hypaphorine, another alkaloid related to the amino acid tryptophan. First reported in the Caribbean sponge Plakortis simplex in 2003, plakohypaphorines A-C were the first iodine-containing compounds based on the...
s from the sponge Plakortis simplex
Homoscleromorpha
Homoscleromorpha is a subclass of marine demosponges containing a single order, Homosclerophorida and a single family, Plakinidae.-Taxonomy:This class has recently been recognised as the fourth major line of sponges....
.


Iodomethane
Methyl iodide, also called iodomethane, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally...
produced by the marine environment, microbial activitiy in rice paddies, and the burning of biological material is estimated to be 214 kilotonnes per year. The volatile iodomethane is broken up by oxidation reactions in the atmosphere and a global iodine cycle is established.
Moreover, iodine can add to double bonds of docosahexaenoic acid
Docosahexaenoic acid
Docosahexaenoic acid is an omega-3 fatty acid that is a primary structural component of the human brain and retina. In chemical structure, DHA is a carboxylic acid with a 22-carbon chain and six cis double bonds; the first double bond is located at the third carbon from the omega end...
and arachidonic acid
Arachidonic acid
Arachidonic acid is a polyunsaturated omega-6 fatty acid 20:4.It is the counterpart to the saturated arachidic acid found in peanut oil, Arachidonic acid (AA, sometimes ARA) is a polyunsaturated omega-6 fatty acid 20:4(ω-6).It is the counterpart to the saturated arachidic acid found in peanut oil,...
of cellular membranes, making iodolipids which seem to have an apoptic action and are less reactive to free oxygen radicals.
Methods for preparation of the C–I bond
Organoiodine compounds are prepared by numerous routes, depending on the degree and regiochemistry of iodination sought and the nature of the precursors. The direct iodination with I2 is employed with unsaturated substrates:- RHC=CH2 + I2 → RHIC-CIH2
The iodide anion is a good nucleophile and will displace chloride, tosylate, bromide and other leaving groups, as in the Finkelstein reaction
Finkelstein reaction
The Finkelstein reaction, named for the German chemist Hans Finkelstein , is an SN2 reaction that involves the exchange of one halogen atom for another...
. Aromatic iodides may be prepared via a diazonium salt in the Sandmeyer reaction
Sandmeyer reaction
The Sandmeyer reaction is a chemical reaction used to synthesize aryl halides from aryl diazonium salts. It is named after the Swiss chemist Traugott Sandmeyer....
.
Because of its low ionization energy, iodine is readily converted to reagents that deliver the equivalent of "I+". A representative electrophilic iodination reagent is iodine monochloride
Iodine monochloride
Iodine monochloride is an interhalogen compound with the formula ICl. It is a red-brown compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, ICl is highly polar and behaves as a source of I+....
.
See also
- Organofluorine compounds
- Organochlorine compounds
- Organobromine compoundOrganobromine compoundOrganobromine compounds are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application is the use of polybrominated diphenyl ethers as fire-retardants. A variety of minor organobromine compounds are found in...
s - PeriodinanePeriodinanePeriodinanes are chemical compounds containing hypervalent iodine. These iodine compounds are hypervalent because the iodine atom in it contains more than the 8 electrons in the valence shell required for the octet rule...
s

