
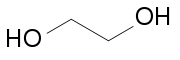
Diol
Encyclopedia
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
containing two hydroxyl groups (—OH groups)
A geminal diol
Geminal diol
A geminal diol is any organic compound having two hydroxyl functional groups bound to the same carbon atom.The simplest geminal diol is methanediol CH4O2 or H2C2...
has two hydroxyl groups bonded to the same atom. Examples include methanediol
Methanediol
Methanediol, also known as formaldehyde monohydrate or methylene glycol, is a chemical compound with chemical formula CH4O2, or H2C2...
H2C(OH)2 and 1,1,1,3,3,3-hexafluoropropane-2,2-diol (F3C)2C(OH)2, the hydrated form of hexafluoroacetone
Hexafluoroacetone
Hexafluoroacetone is a chemical compound with the formula CF3-CO-CF3. It is structurally similar to acetone, however its reactivity is markedly different. It comes in the form of a colourless, hygroscopic, nonflammable, highly reactive gas characterized by a musty odour...
.
A vicinal diol is a diol with two hydroxyl groups in vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
positions, that is, attached to adjacent atoms. Examples include 1,2-ethanediol or ethylene glycol HO—(CH2)2—OH, a common ingredient of antifreeze
Antifreeze
Antifreeze is a freeze preventive used in internal combustion engines and other heat transfer applications, such as HVAC chillers and solar water heaters....
products; and propane-1,2-diol or alpha propylene glycol, HO—CH2—CH(OH)—CH3 used in the food and medicine industry as well as a relatively non-poisonous antifreeze product.
Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol HO—(CH2)4—OH and bisphenol A
Bisphenol A
Bisphenol A is an organic compound with two phenol functional groups. It is used to make polycarbonate plastic and epoxy resins, along with other applications....
, and propylene-1,3-diol or beta propylene glycol
Propylene glycol
Propylene glycol, also called 1,2-propanediol or propane-1,2-diol, is an organic compound with formula C3H8O2 or HO-CH2-CHOH-CH3...
, HO-CH2-CH2-CH2-OH.
Classification
Diols can be:- Linear or branched;
- Aliphatic or aromatic (bisphenol ABisphenol ABisphenol A is an organic compound with two phenol functional groups. It is used to make polycarbonate plastic and epoxy resins, along with other applications....
).
Examples of aliphatic diols:
| Linearity of the diol | Hydroxyls on adjacent carbons (vicinal diols) | Hydroxyls on non-adjacent carbons |
|---|---|---|
| Linear | Ethylene glycol Ethylene glycol Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid... |
1,3-Propanediol 1,3-Propanediol 1,3-Propanediol is the organic compound with the formula CH22. This three-carbon diol is a colorless viscous liquid that is miscible with water.-Products:... , 1,4-Butanediol 1,4-Butanediol 1,4-Butanediol is the organic compound with the formula HOCH2CH2CH2CH2OH. This colorless viscous liquid is derived from butane by placement of alcohol groups at each end of the chain. It is one of four stable isomers of butanediol.-Synthesis:... , 1,5-Pentanediol 1,5-Pentanediol 1,5-Pentanediol is the organic compound with the formula HOCH2CH2CH2CH2CH2OH. Like other diols, this viscous liquid is used as plasticizer and also forms polyesters that are used as emulsifying agents and resin intermediates... , 1,8-octanediol, |
| Branched | 1,2-Propanediol Propylene glycol Propylene glycol, also called 1,2-propanediol or propane-1,2-diol, is an organic compound with formula C3H8O2 or HO-CH2-CHOH-CH3... , 1,2-Butanediol 1,2-Butanediol 1,2-Butanediol is a vic-diol first described by Charles-Adolphe Wurtz in 1859. It is produced industrially as a byproduct of the production of 1,4-butanediol from butadiene, and is also a byproduct of the catalytic hydrocracking of starches and sugars such as sorbitol to ethylene glycol and... , 2,3-Butanediol 2,3-Butanediol 2,3-Butanediol is a chemical compound composed of carbon, hydrogen, and oxygen. Its formula is C4H10O2. It is one of the constitutional isomers of butanediol.... |
1,3-Butanediol 1,3-Butanediol 1,3-Butanediol is an organic chemical, an alcohol. It is commonly used as a solvent for food flavouring agents and is a co-monomer used in certain polyurethane and polyester resins. It is one of four stable isomers of butanediol. In biology, 1,3-butanediol is used as a hypoglycaemic agent.... , 1,2-pentanediol, Etohexadiol Etohexadiol Etohexadiol is an ectoparasiticide.... , p-Menthane-3,8-diol P-menthane-3,8-diol p-Menthane-3,8-diol, also known as para-menthane-3,8-diol, PMD, or Menthoglycol, is an active ingredient used in insect repellents. It smells similar to menthol and acts as a coolant. PMD is found in very small quantities in the essential oil within leaves of the Eucalyptus citriodora tree... , 2-Methyl-2,4-pentanediol 2-Methyl-2,4-pentanediol 2-Methyl-2,4-pentanediol is a chemical used in industrial coatings and as a chemical intermediate. Total European and USA production was 15000 tonnes in 2000... |
Synthesis
Because diols are a common functional groupFunctional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
arrangement, numerous methods of preparation have been developed.
- Vicinal diols can be produced from the oxidation of alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s, usually with dilute acidic potassium permanganatePotassium permanganatePotassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
, also known as potassium manganate(VII). Using alkaline potassium manganate(VII) produces a colour change from clear deep purple to clear green; acidic potassium manganate(VII) turns clear colourless. - Osmium tetroxide can similarly be used to oxidize alkenes to vicinal diols.
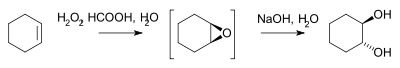
- Hydrogen peroxideHydrogen peroxideHydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
reacts with an alkene to the epoxideEpoxideAn epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
and then by saponificationSaponificationSaponification is a process that produces soap, usually from fats and lye. In technical terms, saponification involves base hydrolysis of triglycerides, which are esters of fatty acids, to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes...
to the diol for example in the synthesis of trans-cyclohexanediol batch or by microreactorMicroreactorA microreactor or microstructured reactor or microchannel reactor is a device in which chemical reactions take place in a confinement with typical lateral dimensions below 1 mm;the most typical form of such confinement are microchannels...
:
- A chemical reaction called Sharpless asymmetric dihydroxylationSharpless asymmetric dihydroxylationSharpless asymmetric dihydroxylation is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol....
can be used to produce chiralChirality (chemistry)A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
diols from alkenes using an osmate reagentReagentA reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
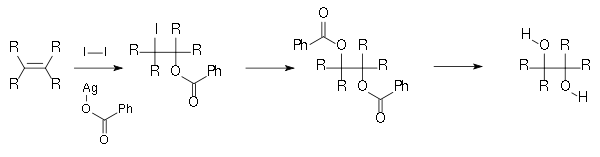
and a chiral catalyst. - Another method is the Woodward cis-hydroxylationWoodward cis-hydroxylationThe Woodward cis-hydroxylation is the chemical reaction of alkenes with iodine and silver acetate in wet acetic acid to form cis-diols. The reaction is named after its discoverer, Robert Burns Woodward....
(cis diol) and the related Prévost reactionPrévost reactionThe Prévost reaction is chemical reaction in which an alkene is converted by iodine and the silver salt of benzoic acid to a vicinal diol with anti stereochemistry...
(anti diol), depicted below, which both use iodine and the silver salt of a carboxylic acid.
- A chemical reaction called Sharpless asymmetric dihydroxylation
- In the Prins reactionPrins reactionThe Prins reaction is an organic reaction consisting of an electrophilic addition of an aldehyde or ketone to an alkene or alkyne followed by capture of a nucleophile. The outcome of the reaction depends on reaction conditions . With water and a protic acid such as sulfuric acid as the reaction...
1,3-diols can be formed in a reaction between an alkeneAlkeneIn organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
and formaldehydeFormaldehydeFormaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
. - Geminal diols can be formed by the hydration of ketones.
- In the Prins reaction
General diols
Diols react as alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, by esterification and ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
formation.
Diols such as ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
are used as co-monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s in polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
reactions forming polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s including some polyester
Polyester
Polyester is a category of polymers which contain the ester functional group in their main chain. Although there are many polyesters, the term "polyester" as a specific material most commonly refers to polyethylene terephthalate...
s and polyurethane
Polyurethane
A polyurethane is any polymer composed of a chain of organic units joined by carbamate links. Polyurethane polymers are formed through step-growth polymerization, by reacting a monomer with another monomer in the presence of a catalyst.Polyurethanes are...
s. A different monomer with two identical functional groups, such as a dioyl dichloride or dioic acid is required to continue the process of polymerization through repeated esterification processes.
A diol can be converted to cyclic ether by using an acid catalyst, this is diol cyclization. Firstly, it involves protonation of the hydroxyl group. Then, followed by intramolecular nucleophilic substitution, the second hydroxyl group attacks the electron deficient carbon. Provided that there are enough carbon atoms that the angle strain is not too much, a cyclic ether can be formed.
Vicinal diols
In glycol cleavageGlycol cleavage
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a (vicinal diol...
, the C-C bond in a vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
diol is cleaved with formation of ketone or aldehyde functional groups. See Diol oxidation.
Geminal diols
In general, organic geminal diols readily dehydrateDehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
to form a carbonyl group. For example, carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
((HO)2C=O) is unstable and has a tendency to convert to carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2) and water (H2O). Nevertheless, in rare situations the chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
is in favor of the geminal diol. For example, when formaldehyde
Formaldehyde
Formaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
(H2C=O) is dissolved in water the geminal diol (H2C(OH)2), methanediol
Methanediol
Methanediol, also known as formaldehyde monohydrate or methylene glycol, is a chemical compound with chemical formula CH4O2, or H2C2...
, is favored. Other examples are the cyclic geminal diols decahydroxycyclopentane
Decahydroxycyclopentane
Decahydroxycyclopentane is an organic compound with formula C5O10H10 or C510. It is a fivefold geminal diol on a cyclopentane backbone.The compound can be regarded as the fivefold hydrate of cyclopentanepentone. Indeed, the product referred to in the literature and trade as "cyclopentanepentone...
(C5(OH)10) and dodecahydroxycyclohexane
Dodecahydroxycyclohexane
Dodecahydroxycyclohexane is an organic compound with molecular formula C6O12H12 or C612. It is a sixfold geminal diol with a cyclohexane backbone and can be regarded as a sixfold hydrate of cyclohexanehexone .-Dihydrate:...
(C6(OH)12), which are stable, whereas the corresponding oxocarbon
Oxocarbon
An oxocarbon or oxide of carbon is an inorganic compound consisting only of carbon and oxygen.The simplest and most common oxocarbons are carbon monoxide and carbon dioxide...
s (C5O5 and C6O6) do not seem to be.
See also
- AlcoholAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, chemical compounds with one hydroxylHydroxylA hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group - TriolTriolA triol is a chemical compound containing three hydroxyl groups , such as glycerol. An example of triol on benzene is benzenetriol.- See also :* Alcohols, chemical compounds with one hydroxyl group...
s, chemical compounds with three hydroxylHydroxylA hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group - PolyolPolyolA polyol is an alcohol containing multiple hydroxyl groups. In two technological disciplines the term "polyol" has a special meaning: food science and polymer chemistry.- Polyols in food science :...
s, chemical compounds with multiple hydroxylHydroxylA hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups - Ethylene glycolEthylene glycolEthylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...

