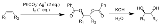
Prévost reaction
Encyclopedia
The Prévost reaction is chemical reaction
in which an alkene
is converted by iodine
and the silver
salt of benzoic acid
to a vicinal
diol
with anti stereochemistry. The reaction was discovered by the French chemist Charles Prévost (1899-1983).
gives another short-lived iodinium salt (3). Nucleophilic substitution (SN2
) by the benzoate salt gives the ester (4). Another silver ion causes the neighboring group substitution of the benzoate ester to gives the oxonium salt
(5). A second SN2 substitution
by the benzoate anion give the desired diester (6).
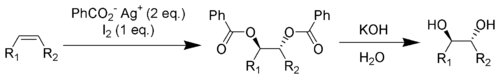
 In the final step hydrolysis
In the final step hydrolysis
of the ester
groups gives the anti-diol. This outcome is the opposite of that of the related Woodward cis-hydroxylation
which gives syn addition
.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
in which an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
is converted by iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
and the silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
salt of benzoic acid
Benzoic acid
Benzoic acid , C7H6O2 , is a colorless crystalline solid and the simplest aromatic carboxylic acid. The name derived from gum benzoin, which was for a long time the only source for benzoic acid. Its salts are used as a food preservative and benzoic acid is an important precursor for the synthesis...
to a vicinal
Vicinal (chemistry)
In chemistry vicinal stands for any two functional groups bonded to two adjacent carbon atoms. For example the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not....
diol
Diol
A diol or glycol is a chemical compound containing two hydroxyl groups A geminal diol has two hydroxyl groups bonded to the same atom...
with anti stereochemistry. The reaction was discovered by the French chemist Charles Prévost (1899-1983).

Reaction mechanism
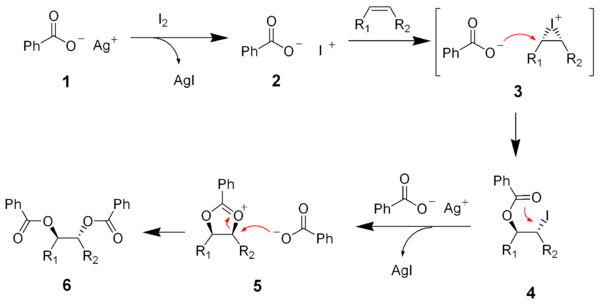
The reaction between silver benzoate (1) and iodine is very fast and produces a very reactive iodinium benzoate intermediate (2). The reaction of the iodinium salt (2) with an alkeneAlkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
gives another short-lived iodinium salt (3). Nucleophilic substitution (SN2
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
) by the benzoate salt gives the ester (4). Another silver ion causes the neighboring group substitution of the benzoate ester to gives the oxonium salt
Oxonium ion
The oxonium ion in chemistry is any oxygen cation with three bonds. The simplest oxonium ion is the hydronium ion H3O+. Another oxonium ion frequently encountered in organic chemistry is obtained by protonation or alkylation of a carbonyl group e.g...
(5). A second SN2 substitution
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
by the benzoate anion give the desired diester (6).

Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of the ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
groups gives the anti-diol. This outcome is the opposite of that of the related Woodward cis-hydroxylation
Woodward cis-hydroxylation
The Woodward cis-hydroxylation is the chemical reaction of alkenes with iodine and silver acetate in wet acetic acid to form cis-diols. The reaction is named after its discoverer, Robert Burns Woodward....
which gives syn addition
Syn addition
In organic chemistry, syn and anti addition are different ways in which two substituents can be added to a double bond or triple bond. This article will use alkenes as examples....
.

