
Syn addition
Encyclopedia
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
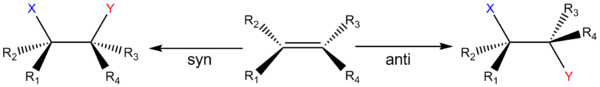
, syn and anti addition are different ways in which two substituents can be added to a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
or triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
. This article will use alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s as examples.
Syn addition is the addition of two substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s to the same side (or face) of a double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
or triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
, resulting in a decrease in bond order
Bond order
Bond order is the number of chemical bonds between a pair of atoms. For example, in diatomic nitrogen N≡N the bond order is 3, while in acetylene H−C≡C−H the bond order between the two carbon atoms is also 3, and the C−H bond order is 1. Bond order gives an indication to the stability of a bond....
but an increase in number of substituents. Generally the substrate
Substrate (chemistry)
In chemistry, a substrate is the chemical species being observed, which reacts with a reagent. This term is highly context-dependent. In particular, in biochemistry, an enzyme substrate is the material upon which an enzyme acts....
will be an alkene or alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
. An example of syn addition would be the oxidation of an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
to a diol via a suitable oxidizing agent such as Osmium tetroxide OsO4 or Potassium permanganate
Potassium permanganate
Potassium permanganate is an inorganic chemical compound with the formula KMnO4. It is a salt consisting of K+ and MnO4− ions. Formerly known as permanganate of potash or Condy's crystals, it is a strong oxidizing agent. It dissolves in water to give intensely purple solutions, the...
KMnO4.
Anti addition is in direct contrast to syn addition. In anti addition, two substituents are added to opposite sides (or faces) of a double bond or triple bond, once again resulting in a decrease in bond order but an increase in number of substituents. The classical example of this is bromination (any halogenation
Halogenation
Halogenation is a chemical reaction that incorporates a halogen atom into a molecule in substitution of hydrogen atom. Halogenation takes place in the gas phase. There are four types of halogenation: fluorination, chlorination, bromination, and iodination...
) of alkenes.
Depending on the substrate double bond, addition can have different effects on the molecule. After addition to a straight-chain
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
alkene such as C2H4, the resulting alkane will rapidly and freely rotate around its single sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
under normal conditions (i.e. room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
). Thus whether substituents are added to the same side (syn) or opposite sides (anti) of a double can usually be ignored due to free rotation. However, if chirality
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
or the specific absolute orientation of the substituents needs to be taken into account, knowing the type of addition is significant. Unlike straight-chain alkenes, cycloalkene
Cycloalkane
Cycloalkanes are types of alkanes that have one or more rings of carbon atoms in the chemical structure of their molecules. Alkanes are types of organic hydrocarbon compounds that have only single chemical bonds in their chemical structure...
syn addition allows stable addition of substituents to the same side of the ring, where they remain together. The cyclic locked ring structure prevents free rotation.
Syn elimination and anti elimination are the reverse processes of syn and anti addition. These result in a new double bond, such as in Ei elimination
Ei mechanism
Ei elimination in organic chemistry is a special type of elimination reaction in which two vicinal substituents on an alkane framework leave simultaneously in a single step to form an alkene in a syn elimination . In regular eliminations this reaction would involve a base or would in many cases...
.

