
Cycloalkane
Encyclopedia
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
s that have one or more rings of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s in the chemical structure
Chemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
of their molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s. Alkanes are types of organic
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
compounds
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
that have only single chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s in their chemical structure. Cycloalkanes consist of only carbon (C) and hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(H) atoms and are saturated because there are no multiple C-C bonds to hydrogenate
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
(add more hydrogen to). A general chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
for cycloalkanes would be CnH2(n+1-g) where n = number of C atoms and g = number of rings in the molecule. Cycloalkanes with a single ring are named analogously to their normal alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
counterpart of the same carbon count: cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
, cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
, cyclopentane
Cyclopentane
Cyclopentane is a highly flammable alicyclic hydrocarbon with chemical formula 510 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C...
, cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
, etc. The larger cycloalkanes, with greater than 20 carbon atoms are typically called cycloparaffins.
Cycloalkanes are classified into small, common, medium, and large cycloalkanes, where cyclopropane and cyclobutane are the small ones, cyclopentane, cyclohexane, cycloheptane are the common ones, cyclooctane through cyclotridecane are the medium ones, and the rest are the larger ones.
Nomenclature
- See also: IUPAC nomenclatureIUPAC nomenclatureA chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry ....
Spiro compound
A spiro compound is a bicyclic organic compound with rings connected through just one atom. The rings can be different in nature or identical. The connecting atom is also called the spiroatom, most often a quaternary carbon...
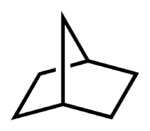
alkanes is more complex, with the base name indicating the number of carbons in the ring system, a prefix indicating the number of rings (e.g., "bicyclo"), and a numeric prefix before that indicating the number of carbons in each part of each ring, exclusive of vertices. For instance, a bicyclooctane that consists of a six-member ring and a four-member ring, which share two adjacent carbon atoms that form a shared edge, is [4.2.0]-bicyclooctane. That part of the six-member ring, exclusive of the shared edge has 4 carbons. That part of the four-member ring, exclusive of the shared edge, has 2 carbons. The edge itself, exclusive of the two vertices that define it, has 0 carbons.
There is more than one convention (method or nomenclature) for the naming of compounds, which can be confusing for those who are just learning, and inconvenient for those who are well rehearsed in the older ways. For beginners it is best to learn IUPAC nomenclature from a source that is up to date, because this system is constantly being revised. In the above example [4.2.0]-bicyclooctane would be written bicyclo[4.2.0]octane to fit the conventions for IUPAC naming. It has then got room for an additional numerical prefix if there is the need to include details of other attachments to the molecule such as chlorine or a methyl group. Another convention for the naming of compounds is the common name, which is a shorter name and it gives less information about the compound. An example of a common name is terpineol
Terpineol
Terpineol is a naturally occurring monoterpene alcohol that has been isolated from a variety of sources such as cajuput oil, pine oil, and petitgrain oil. There are three isomers, alpha-, beta-, and gamma-terpineol, the last two differing only by the location of the double bond...
, the name of which can tell us only that it is an alcohol (because the suffix 'ol' is in the name) and it should then have a hydroxide (OH) group attached to it.
An example of the IUPAC method is given in the image to the right. In this example the base name is listed first, which indicates the total number of carbons in both rings including the carbons making up the shared edge (e.g., heptane, which means hept or 7 carbons, and ane, which indicates only single bonding between carbons). Then in front of the base name is the numerical prefix, which lists the number of carbons in each ring, excluding the carbons that are shared by each ring, plus the number of carbons on the bridge between the rings. In this case there are two rings with two carbons each and a single bridge with one carbon, excluding the carbons shared by it and the other two rings. There is a total of three numbers and they are listed in descending order separated by dots, thus: [2.2.1].
Before the numerical prefix is another prefix indicating the number of rings (e.g., "bicyclo"). Thus, the name is bicyclo[2.2.1]heptane.
The group of cycloalkanes are also known as naphthenes, as they are compounds of petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
or naphtha
Naphtha
Naphtha normally refers to a number of different flammable liquid mixtures of hydrocarbons, i.e., a component of natural gas condensate or a distillation product from petroleum, coal tar or peat boiling in a certain range and containing certain hydrocarbons. It is a broad term covering among the...
.
Properties
Cycloalkanes are similar to alkanes in their general physical properties, but they have higher boiling pointBoiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
s, melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
s, and densities
Density
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
than alkanes. This is due to stronger London forces because the ring shape allows for a larger area of contact. Containing only C-C and C-H bonds, unreactivity of cycloalkanes with little or no ring strain (see below) are comparable to non-cyclic alkanes.
Ring strain
The carbon atoms in cycloalkanes are sp3 hybridized and are therefore a deviation from the ideal tetrahedral bond angles of 109°28'. This causes an increase in potential energy and an overall destabilizing effect. Eclipsing of hydrogen atoms is an important destabilizing effect, as well. The strain energy of a cycloalkane is the theoretical increase in energy caused by the compound's geometry, and is calculated by comparing the experimental standard enthalpy change of combustionStandard enthalpy change of combustion
The standard enthalpy of combustion is the enthalpy change when one mole of a substance completely reacts with oxygen under standard thermodynamic conditions...
of the cycloalkane with the value calculated using average bond energies.
Ring strain is highest for cyclopropane
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
, in which the carbon atoms form a triangle and therefore have 60 degree C-C-C bond angles. There are also three pairs of eclipsed hydrogens. The ring strain is calculated to be around 120 kJ/mol.
Cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
has the carbon atoms in a puckered square with approximately 90-degree bond angles; "puckering" reduces the eclipsing interactions between hydrogen atoms. Its ring strain is therefore slightly less, at around 110 kJ/mol.
For a theoretical planar cyclopentane
Cyclopentane
Cyclopentane is a highly flammable alicyclic hydrocarbon with chemical formula 510 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and below the plane. It occurs as a colorless liquid with a petrol-like odor. Its melting point is −94 °C...
the C-C-C bond angles would be 108 degrees, very close to the measure of the tetrahedral angle. Actual cyclopentane molecules are puckered, but this changes only the bond angles slightly so that angle strain is relatively small. The eclipsing interactions are also reduced, leaving a ring strain of about 25 kJ/mol.
In cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
the ring strain and eclipsing interactions are negligible because the puckering of the ring allows ideal tetrahedral bond angles to be achieved. As well, in the most stable chair form of cyclohexane, axial hydrogens on adjacent carbon atoms are pointed in opposite directions, virtually eliminating eclipsing strain.
After cyclohexane, the molecules are unable to take a structure with no ring strain, resulting in an increase in strain energy, which peaks at 9 carbons (around 50 kJ/mol). After that, strain energy slowly decreases until 12 carbon atoms, where it drops significantly; at 14, another significant drop occurs and the strain is on a level comparable with 10 kJ/mol. After 14 carbon atoms, sources disagree on what happens to ring strain, some indicating that it increases steadily, others saying that it disappears entirely. However, bond angle strain and eclipsing strain are an issue only for smaller rings.
Reactions
The simple and the bigger cycloalkanes are very stable, like alkanes, and their reactions, for example, radical chain reactionsRadical substitution
In organic chemistry, a radical substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.The reaction always involves at least two steps, and possibly a third....
, are like alkanes.
The small cycloalkanes - in particular, cyclopropane - have a lower stability due to Baeyer strain
Baeyer strain theory
Baeyer strain theory or strain theory explains specific behaviour of chemical compounds in terms of bond angle strain.It was proposed by Adolf von Baeyer in 1885 to account for the unusual chemical reactivity in ring-opening reactions of cyclopropanes and cyclobutanes where this angle strain is...
and ring strain
Ring strain
In organic chemistry, ring strain is the tendency of a cyclic molecule, such as cyclopropane, to destabilize when its atoms are in non-favorable high energy spatial orientations...
. They react similarly to alkenes, though they do not react in electrophilic addition
Electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed...
, but in nucleophilic aliphatic substitution. These reactions are ring-opening reactions or ring-cleavage reactions of alkyl cycloalkane
Alkyl cycloalkane
Alkyl cycloalkanes are chemical compounds with an alkyl group with a single ring of carbons to which hydrogens are attached according to the formula...
s. Cycloalkanes can be formed in a Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
followed by a catalytic hydrogenation.
External links
- "Cycloalkanes" at the online Encyclopædia BritannicaEncyclopædia BritannicaThe Encyclopædia Britannica , published by Encyclopædia Britannica, Inc., is a general knowledge English-language encyclopaedia that is available in print, as a DVD, and on the Internet. It is written and continuously updated by about 100 full-time editors and more than 4,000 expert...
- "Cycloalkanes" by the Chemistry staff at Westminster CollegeWestminster College, PennsylvaniaWestminster College is a liberal arts college located in New Wilmington, Pennsylvania, United States. Founded in 1852, it is affiliated with the Presbyterian Church...

