Dehydration reaction
Encyclopedia
In chemistry
and the biological sciences, a dehydration reaction is usually defined as a chemical reaction
that involves the loss of water
from the reacting molecule. Dehydration reactions are a subset of elimination reaction
s. Because the hydroxyl
group (-OH) is a poor leaving group
, having a Brønsted acid catalyst often helps by protonating the hydroxyl group to give the better leaving group, -OH2+. The reverse of a dehydration reaction is a hydration reaction
.
Dehydration reactions and dehydration synthesis have the same meaning, and are often used interchangeably. Two monosaccharide
s, such as glucose
and fructose
, can be joined together (to form sucrose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide
.
The process of hydrolysis
is the reverse reaction, meaning that the water is recombined with the two hydroxyl groups and the disaccharide reverts to being monosaccharides.
In the related condensation reaction
water is released from two different reactants.
, there are many examples of dehydration reactions for example dehydration of alcohols or sugars.
Some dehydration reactions can be mechanistically complex, for instance the reaction of a sugar
(sucrose) with concentrated sulfuric acid
: to form carbon involves formation of carbon carbon bonds.
The reaction is driven by the strongly exothermic reaction sulfuric acid has with water.
Common dehydrating agents; concentrated sulfuric acid
, concentrated phosphoric acid
, hot aluminium oxide
, hot ceramic.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
and the biological sciences, a dehydration reaction is usually defined as a chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
that involves the loss of water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
from the reacting molecule. Dehydration reactions are a subset of elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
s. Because the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group (-OH) is a poor leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
, having a Brønsted acid catalyst often helps by protonating the hydroxyl group to give the better leaving group, -OH2+. The reverse of a dehydration reaction is a hydration reaction
Hydration reaction
In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous...
.
Dehydration reactions and dehydration synthesis have the same meaning, and are often used interchangeably. Two monosaccharide
Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s, such as glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
, can be joined together (to form sucrose) using dehydration synthesis. The new molecule, consisting of two monosaccharides, is called a disaccharide
Disaccharide
A disaccharide or biose is the carbohydrate formed when two monosaccharides undergo a condensation reaction which involves the elimination of a small molecule, such as water, from the functional groups only. Like monosaccharides, disaccharides form an aqueous solution when dissolved in water...
.
The process of hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
is the reverse reaction, meaning that the water is recombined with the two hydroxyl groups and the disaccharide reverts to being monosaccharides.
In the related condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
water is released from two different reactants.
Dehydration reactions
In organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, there are many examples of dehydration reactions for example dehydration of alcohols or sugars.
| Reaction | Equation | | |
|---|---|---|
| Conversion of alcohol Alcohol In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms.... s to ether Ether Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"... s |
2 R-OH → R-O-R + H2O | |
| Conversion of alcohols to alkene Alkene In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond... s |
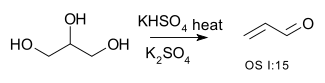
R-CH2-CHOH-R → R-CH=CH-R + H2O | for example the conversion of glycerol Glycerol Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids... to acrolein Acrolein Acrolein is the simplest unsaturated aldehyde. It is produced widely but is most often immediately reacted with other products due to its instability and toxicity... : or the dehydration of 2-methyl-1-cyclohexanol to (mainly) 1-methylcyclohexene |
| Conversion of carboxylic acid Carboxylic acid Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group... s to acid anhydrides |
2 RCO2H → (RCO)2O + H2O | |
| Conversion of amide Amide In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an... s to nitrile Nitrile A nitrile is any organic compound that has a -C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, one example being super glue .Inorganic compounds containing the -C≡N group are not called... s |
RCONH2 → R-CN + H2O | |
| dienol benzene rearrangement | 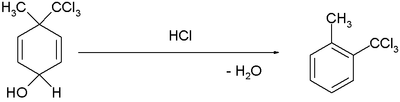 |
|
| Dehydration reactions | ||
Some dehydration reactions can be mechanistically complex, for instance the reaction of a sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
(sucrose) with concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
: to form carbon involves formation of carbon carbon bonds.
- C12H22O11 + 98% Sulfuric acid → 12 C (graphitic foam) + 11 H2O steam + Sulfuric acid/water mixture
The reaction is driven by the strongly exothermic reaction sulfuric acid has with water.
Common dehydrating agents; concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, concentrated phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
, hot aluminium oxide
Aluminium oxide
Aluminium oxide is an amphoteric oxide with the chemical formula 23. It is commonly referred to as alumina, or corundum in its crystalline form, as well as many other names, reflecting its widespread occurrence in nature and industry...
, hot ceramic.