
Elimination reaction
Encyclopedia
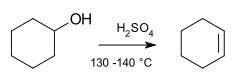
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which two substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
s are removed from a molecule in either a one or two-step mechanism. Either the unsaturation of the molecule increases (as in most organic elimination reactions) or the valence of an atom in the molecule decreases by two, a process known as reductive elimination.
An important class of elimination reactions are those involving alkyl halides, or alkanes in general, with good leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
s, reacting with a Lewis base to form an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
in the reverse of an addition reaction
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
. When the substrate is asymmetric, regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
is determined by Zaitsev's rule
Zaitsev's rule
In chemistry, Zaitsev's rule, Saytzeff's rule or Saytsev's rule named after Alexander Mikhailovich Zaitsev is a rule that states that if more than one alkene can be formed during dehalogenation by an elimination reaction, the more stable alkene is the major product...
. The one and two-step mechanisms are named and known as E2 reaction and E1 reaction, respectively.
E2 mechanism
In the 1920s, Sir Christopher IngoldChristopher Kelk Ingold
Sir Christopher Kelk Ingold FRS was a British chemist based in Leeds and London. His groundbreaking work in the 1920s and 1930s on reaction mechanisms and the electronic structure of organic compounds was responsible for the introduction into mainstream chemistry of concepts such as nucleophile,...
proposed a model to explain a peculiar type of chemical reaction: the E2 mechanism. E2 stands for bimolecular elimination and has the following specificities.
- It is a one-step process of elimination with a single transition stateTransition stateThe transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
. - Typical of primary or secondary substituted alkyl halides.
- The reaction rateReaction rateThe reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
, influenced by both the alkyl halide and the base, is second order. - Because E2 mechanism results in formation of a pi bond, the two leaving groups (often a hydrogen and a halogenHalogenThe halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
) need to be coplanar. An antiperiplanar transition stateTransition stateThe transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
has staggeredStaggeredIn organic chemistry, a staggered conformation is a chemical conformation of an ethane-like moiety abcX-Ydef in which the substituents a,b,and c are at the maximum distance from d,e,and f...
conformation with lower energy and a synperiplanar transition state is in eclipsedEclipsedIn chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X-A-B-Y is 0°. Such a conformation exists in any open chain single chemical bond connecting two sp3 hybridised atoms, and is...
conformation with higher energy. The reaction mechanism involving staggered conformation is more favourable for E2 reactions. - Reaction often present with strong baseBase (chemistry)For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
. - In order for the pi bond to be created, the hybridization of carbons need to be lowered from sp3 to sp2.
- The C-H bond is weakened in the rate determining step and therefore the deuterium isotope effect is larger than 1.
- This reaction type has similarities with the SN2 reactionSN2 reactionThe SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
mechanism.
The reaction fundamental elements are
- Breaking of the carbon-hydrogen and carbon-halogen bonds in one step.
- Formation of a C=C Pi bondMolecular geometryMolecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
.
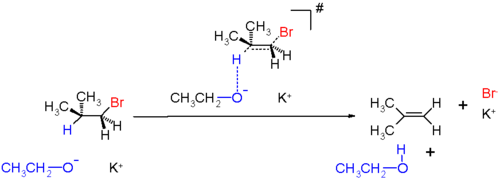
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
ethoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
. The reaction products are isobutylene
Isobutylene
Isobutylene is a hydrocarbon of significant industrial importance. It is a four-carbon branched alkene , one of the four isomers of butylene. At standard temperature and pressure it is a colorless flammable gas.-Uses:...
, ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
and potassium bromide
Potassium bromide
Potassium bromide is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the United States. Its action is due to the bromide ion...
.
E1 mechanism
E1 is a model to explain a particular type of chemical elimination reaction. E1 stands for unimolecular elimination and has the following specificities.- It is a two-step process of elimination: ionization and deprotonation.
- IonizationIonizationIonization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or other ions. This is often confused with dissociation. A substance may dissociate without necessarily producing ions. As an example, the molecules of table sugar...
: the carbon-halogen bond breaks to give a carbocationCarbocationA carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
intermediate. - DeprotonationDeprotonationDeprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
of the carbocation.
- Ionization
- Typical of tertiary and some secondary substituted alkyl halides.
- The reaction rateReaction rateThe reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
is influenced only by the concentration of the alkyl halide because carbocation formation is the slowest, rate-determining stepRate-determining stepThe rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
. Therefore first-order kineticsFirst-order reactionFirst-order reaction may refer to:* Order of reaction, in chemical kinetics, the power to which the concentration term of a certain reactant in the rate equation is raised...
apply. - Reaction mostly occurs in complete absence of base or presence of only a weak base.
- E1 reactions are in competition with SN1 reactionSN1 reactionThe SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
s because they share a common carbocationic intermediate. - A deuterium isotope effect is absent.
- No antiperiplanar requirement. An example is the pyrolysisPyrolysisPyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
of a certain sulfonate ester of mentholMentholMenthol is an organic compound made synthetically or obtained from peppermint or other mint oils. It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is -menthol, which is assigned...
:

- Only reaction product A results from antiperiplanar elimination, the presence of product B is an indication that an E1 mechanism is occurring.
- Accompanied by carbocationic rearrangement reactionRearrangement reactionA rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
s
- Accompanied by carbocationic rearrangement reaction
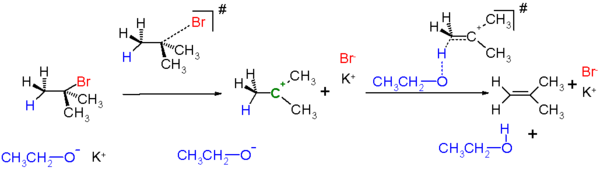
E1 eliminations happen with highly substituted alkyl halides due to 2 main reasons.
- Highly substituted alkyl halides are bulky, limiting the room for the E2 one-step mechanism; therefore, the two-step E1 mechanism is favored.
- Highly substituted carbocations are more stable than methyl or primary substituted. Such stability gives time for the two-step E1 mechanism to occur.
If SN1 and E1 pathways are competing, the E1 pathway can be favored by increasing the heat.
E2 and E1 elimination final notes
The reaction rateReaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
is influenced by halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
's reactivity; iodide
Iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. This page is for the iodide ion and its salts. For information on organoiodides, see organohalides. In everyday life, iodide is most commonly encountered as a component of iodized salt,...
and bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
being favored. Fluoride is not a good leaving group.
There is a certain level of competition between elimination reaction and nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
. More precisely, there are competitions between E2 and SN2
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
and also between E1 and SN1
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
. Substitution generally predominates and elimination occurs only during precise circumstances. Generally, elimination is favored over substitution when
- steric hindrance increases
- basicity increases
- temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
increases - the steric bulk of the base increases (such as in Potassium tert-butoxidePotassium tert-butoxidePotassium tert-butoxide is the chemical compound with the formula 3COK. This colourless solid is a strong base useful in organic synthesis. It exists as a tetrameric cubane-like cluster...
) - the nucleophileNucleophileA nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
is poor
In one study the kinetic isotope effect
Kinetic isotope effect
The kinetic isotope effect is the ratio of reaction rates of two different isotopically labeled molecules in a chemical reaction. It is also called "isotope fractionation," although this term is somewhat broader in meaning...
(KIE) was determined for the gas phase reaction of several alkyl halides with the chlorate
Chlorate
The chlorate anion has the formula ClO. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by a roman numeral in parentheses, e.g...
ion. In accordance with a E2 elimination the reaction with t-butyl chloride results in a KIE of 2.3. The methyl chloride reaction (only SN2 possible) on the other hand has a KIE of 0.85 consistent with a SN2 reaction because in this reaction type the C-H bonds tighten in the transition state. The KIE's for the ethyl (0.99) and isopropyl (1.72) analogues suggest competition between the two reaction modes..
Specific elimination reactions
The E1cB elimination reactionE1cB elimination reaction
The E1cB elimination reaction is a special type of elimination reaction in organic chemistry. This reaction mechanism explains the formation of alkenes from alkyl halides through a carbanion intermediate given specified reaction conditions and specified substrates. The abbreviation stands for ...
is a special type of elimination reaction involving carbanions. In an addition-elimination reaction elimination takes place after an initial addition reaction and in the Ei mechanism
Ei mechanism
Ei elimination in organic chemistry is a special type of elimination reaction in which two vicinal substituents on an alkane framework leave simultaneously in a single step to form an alkene in a syn elimination . In regular eliminations this reaction would involve a base or would in many cases...
both substituents leave simultaneously in a syn addition.
In each of these elimination reactions the reactants have specific leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
s:
- dehydrohalogenationDehydrohalogenationDehydrohalogenation is an organic reaction from which an alkene is obtained from an alkyl halide . It is also called a β-Elimination reaction and is a type of elimination reaction....
, leaving group a halide. - the dehydration reactionDehydration reactionIn chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
is one where the leaving group is water. - the Bamford-Stevens reactionBamford-Stevens reactionThe Bamford–Stevens reaction is a chemical reaction whereby treatment of tosylhydrazones with strong base gives alkenes. It is named for the British chemist William Randall Bamford and the Scottish chemist Thomas Stevens Stevens...
with a tosylhydrazoneTosylhydrazoneA tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.-Synthesis:...
leaving group assisted by alkoxideAlkoxideAn alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands... - the Cope reactionCope reactionThe Cope reaction or Cope elimination, developed by Arthur C. Cope, is an elimination reaction of an amine oxide to form an alkene and a hydroxylamine. The reaction mechanism involves an intramolcular 5-membered cyclic transition state, leading to a syn elimination product...
with an amine oxideAmine oxideAn amine oxide, also known as amine-N-oxide and N-oxide, is a chemical compound that contains the functional group R3N+-O−, an N-O bond with three additional hydrogen and/or hydrocarbon side chains attached to N. Sometimes it is written as R3N→O or, wrongly, as R3N=O.In the strict sense the...
leaving group - the Hofmann eliminationHofmann eliminationHofmann elimination is a process where an amine is reacted to create a tertiary amine and an alkene by treatment with excess methyl iodide followed by treatment with silver oxide, water, and heat.After the first step, a quaternary ammonium iodide salt is created...
with quaternary amine leaving group - the Chugaev reaction with a methyl xanthate leaving group
- the Grieco eliminationGrieco eliminationThe Grieco elimination is an organic reaction describing the elimination reaction of an aliphatic primary alcohol through a selenide to a terminal alkene ....
with a selenoxide leaving group - the Shapiro reactionShapiro reactionThe Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
with a tosylhydrazoneTosylhydrazoneA tosylhydrazone in organic chemistry is a functional group with the general structure RR'C=NH-Ts where Ts is a tosyl group. Organic compounds having this functional group can be accessed by reaction of an aldehyde or ketone with tosylhydrazine.-Synthesis:...
leaving group assisted by alkyllithium - Hydrazone iodinationHydrazone iodinationHydrazone iodination is an organic reaction in which a hydrazone is converted into a vinyl iodide by reaction of iodine and a non-nucleophilic base such as DBU. First published by D. H. R...
with a hydrazoneHydrazoneHydrazones are a class of organic compounds with the structure R1R2C=NNH2. They are related to ketones and aldehydes by the replacement of the oxygen with the NNH2 functional group...
leaving group assisted by iodine - A Grob fragmentationGrob fragmentationA Grob fragmentation, named for the British chemist Cyril A. Grob, is an elimination reaction taking place when an electrofuge and nucleofuge are situated in positions 1 and 3 on an aliphatic chain...
with degree of unsaturation increasing in one of the leaving groups. - the Kornblum–DeLaMare rearrangementKornblum–DeLaMare rearrangementThe Kornblum–DeLaMare rearrangement is a rearrangement reaction in organic chemistry in which a primary or secondary organic peroxide is converted to the corresponding ketone and alcohol under base catalysis...
(elimination over a (H)C-O(OR) bond) with an alcohol leaving group forming a ketone - the Takai olefinationTakai olefinationTakai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. In the original 1986 publication the aldehyde is benzaldehyde and the organochromium species is generated from iodoform or bromoform and an excess of chromium...
with two bulky chromium groups.
See also
- Important publications in organic chemistry
- Arrow pushingArrow pushingArrow pushing or electron pushing is a technique used to describe the progression of organic chemistry reaction mechanisms. In using arrow pushing, "curved arrows" or "curly arrows" are superimposed over the structural formulae of reactants in a chemical equation to show the reaction mechanism...

