Carbocation
Encyclopedia

Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
with a positively-charged carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s in its outer valence shell instead of the eight valence electrons that ensures maximum stability (octet rule
Octet rule
The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low The octet rule is a chemical rule of thumb that states that atoms of low (The octet rule is a chemical rule of thumb that states that atoms of low (...
). Therefore carbocations are often reactive, seeking to fill the octet of valence electrons as well as regain a neutral charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
. One could reasonably assume a carbocation to have sp3 hybridization with an empty sp3 orbital giving positive charge. However, the reactivity of a carbocation more closely resembles sp2 hybridization
Orbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
with a trigonal planar
Trigonal planar
In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of a triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands are identical and all bond angles are 120°. Such species belong to...
molecular geometry.
Example:-Methyl carbocation(CH3+)
Definitions
A carbocation was previously often called a carbonium ion but questions arose on the exact meaning. In present day chemistry a carbocation is any positively charged carbon atom. Two special types have been suggested: carbenium ionCarbenium ion
A carbenium ion is a carbocation of the trivalent and classical type R3C+. It is one of two types of carbocation, the other being a carbonium ion. In older literature a carbocation of the type R3C+ may still be referred to as a carbonium ion, a term that is used now for five-coordinate carbon...
s are trivalent and carbonium ion
Carbonium ion
A carbonium ion is a carbocation of the penta- or tetracoordinated nonclassical type such as an ion of the type R5C+.- Methanium:The parent compound methanium or CH5+ is protonated methane and a superacid. This ion exists as a reactive intermediate in the interstellar medium and can be produced in...
s are pentavalent or hexavalent. University level textbooks only discuss carbocations as if they are carbenium ions, or discuss carbocations with a fleeting reference to the older phrase of carbonium ion or carbenium and carbonium ions. One textbook to this day clings on to the older name of carbonium ion for carbenium ion and reserves the phrase hypervalent carbenium ion for CH5+.
History
The history of carbocations dates back to 1891 when G. Merling reported that he added bromine to tropylidene (cycloheptatrieneCycloheptatriene
Cycloheptatriene is an organic compound with the formula C7H8. This colourless liquid has been of recurring theoretical interest in organic chemistry. It is a ligand in organometallic chemistry and as a building block in organic synthesis...
) and then heated the product to obtain a crystalline, water soluble material, . He did not suggest a structure for it; however Doering
William von Eggers Doering
William von Eggers Doering was a Professor Emeritus at Harvard University and the former Chair of its Chemistry Department...
and Knox convincingly showed that it was tropylium (cycloheptatrienylium) bromide. This ion is predicted to be aromatic by the Hückel Rule
Hückel's rule
In organic chemistry, Hückel's rule estimates whether a planar ring molecule will have aromatic properties. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931...
.
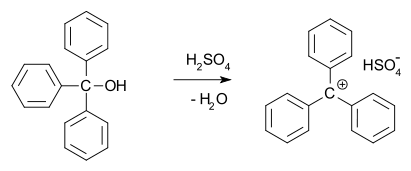
In 1902, Norris and Kehrman independently discovered that colorless triphenylmethanol
Triphenylmethanol
Triphenylmethanol is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene...
gave deep yellow solutions in concentrated sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
. Triphenylmethyl chloride similarly formed orange complexes with aluminium and tin chlorides. In 1902, Adolf von Baeyer
Adolf von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer was a German chemist who synthesized indigo, and was the 1905 recipient of the Nobel Prize in Chemistry. Born in Berlin, he initially studied mathematics and physics at Berlin University before moving to Heidelberg to study chemistry with Robert Bunsen...
recognized the salt-like character of the compounds formed.
Malachite green
Malachite green is an organic compound that is used as a dyestuff and has emerged as a controversial agent in aquaculture. Malachite green is traditionally used as a dye for materials such as silk, leather, and paper...
is a prime example.
Carbocations are reactive intermediates in many organic reactions. This idea, first proposed by Julius Stieglitz in 1899 was further developed by Hans Meerwein in his 1922 study of the Wagner-Meerwein rearrangement
Wagner-Meerwein rearrangement
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon.Several reviews have been published....
. Carbocations were also found to be involved in the SN1 reaction
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
, the E1 reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
, and in rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
s such as the Whitmore 1,2 shift. The chemical establishment was reluctant to accept the notion of a carbocation and for a long time the Journal of the American Chemical Society refused articles that mentioned them.
The first NMR spectrum of a stable carbocation in solution was published by Doering et al. in 1958. It was the heptamethylbenzenonium ion, made by treating hexamethylbenzene
Hexamethylbenzene
Hexamethylbenzene is white crystalline solid with chemical formula C66. It is a synthetic aromatic hydrocarbon with six methyl groups stemming from the carbon centres of the ring. Hexamethylbenzene has historical significance in the field of X-ray crystallography...
with methyl chloride and aluminium chloride
Aluminium chloride
Aluminium chloride is the main compound of aluminium and chlorine. It is white, but samples are often contaminated with iron trichloride, giving it a yellow colour. The solid has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large...
. The stable 7-norbornadienyl cation was prepared by Story et al. in 1960 by reacting norbornadienyl chloride
Norbornadiene
Norbornadiene is an organic compound. This bicyclic hydrocarbon is the most stable diolefin derived from the norbornane and norbornene. Norbornadiene is primarily of interest as a ligand in homogeneous catalysis, but it has been heavily studied due to its high reactivity and distinctive...
with silver tetrafluoroborate
Silver tetrafluoroborate
Silver tetrafluoroborate sometimes referred to "silver BF-4" is an inorganic compound commonly encountered in inorganic and organometallic chemistry. Similar to silver hexafluorophosphate, it is commonly used to replace halide anions or ligands with the weakly coordinating tetrafluoroborate anions...
in sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
at −80 °C. The NMR spectrum established that it was non-classically bridged (the first stable non-classical ion
Non-classical ion
Non-classical ions in organic chemistry are a special type of carbonium ions displaying delocalization of sigma bonds in 3-center-2-electron bonds of bridged systems. The term non-classical ion was first used by John D...
observed).
In 1962, Olah
George Andrew Olah
George Andrew Olah is an American chemist. His research involves the generation and reactivity of carbocations via superacids. For this research, Olah was awarded a Nobel Prize in Chemistry in 1994...
directly observed the tert-butyl carbocation by nuclear magnetic resonance
Nuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
as a stable species on dissolving tert-butyl fluoride in magic acid
Magic acid
Magic acid , is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfonic acid and antimony pentafluoride...
. The NMR of the norbornyl cation was first reported by Schleyer et al. and it was shown to undergo proton-scrambling over a barrier by Saunders et al.
Properties
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, a carbocation is often the target of nucleophilic attack by nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s like hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
(OH−) ions or halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
ions.
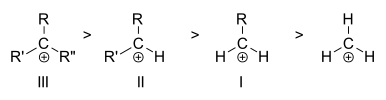
Carbocations are classified as primary, secondary, or tertiary depending on the number of carbon atoms bonded to the ionized carbon. Primary carbocations have one or zero carbons attached to the ionized carbon, secondary carbocations have two carbons attached to the ionized carbon, and tertiary carbocations have three carbons attached to the ionized carbon.
Stability of the carbocation increases with the number of alkyl groups bonded to the charge-bearing carbon. Tertiary carbocations are more stable (and form more readily) than secondary carbocations; primary carbocations are highly unstable because, while ionized higher-order carbons are stabilized by hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
, unsubstituted (primary) carbons are not. Therefore, reactions such as the SN1 reaction
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
and the E1 elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
normally do not occur if a primary carbocation would be formed. An exception to this occurs when there is a carbon-carbon double bond next to the ionized carbon. Such cations as allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
cation CH2=CH–CH2+ and benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
cation C6H5–CH2+ are more stable than most other carbocations. Molecules which can form allyl or benzyl carbocations are especially reactive.
Carbocations undergo rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
s from less stable structures to equally stable or more stable ones with rate constants in excess of 109/sec. This fact complicates synthetic pathways to many compounds. For example, when 3-pentanol is heated with aqueous HCl, the initially formed 3-pentyl carbocation rearranges to a statistical mixture of the 3-pentyl and 2-pentyl. These cations react with chloride ion to produce about 1/3 3-chloropentane and 2/3 2-chloropentane.
Some carbocations such as the norbornyl cation
Norbornane
Norbornane is an organic compound and a saturated hydrocarbon with chemical formula C7H12. It is a crystalline compound with melting point 88 °C. The carbon skeleton is a cyclohexane ring bridged by a methylene group in the 1,4- position, and is a bridged bicyclic compound...
exhibit more or less symmetrical three centre bonding. Cations of this sort have been referred to as non-classical ion
Non-classical ion
Non-classical ions in organic chemistry are a special type of carbonium ions displaying delocalization of sigma bonds in 3-center-2-electron bonds of bridged systems. The term non-classical ion was first used by John D...
s. The energy difference between "classical" carbocations and "non-classical" isomers is often very small, and there is generally little, if any activation energy involved in the transition between "classical" and "non-classical" structures. The "non-classical" form of the 2-butyl carbocation is essentially 2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
with a proton directly above the centre of what would be the carbon-carbon double bond. "Non-classical" carbocations were once the subject of great controversy. One of George Olah's greatest contributions to chemistry was resolving this controversy.
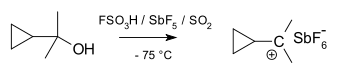
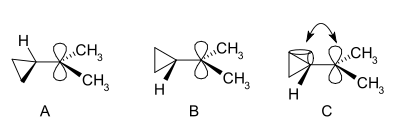
Specific carbocations
Cyclopropylcarbinyl cations can be studied by NMR:In the NMR spectrum of a dimethyl derivative, two nonequivalent signals are found for the two methyl groups indicating that the molecular conformation of this cation not perpendicular (as in A) but is bisected (as in B) with the empty p-orbital and the cyclopropyl ring system in the same plane:
In terms of bent bond theory, this preference is explained by assuming favorable orbital overlap
Orbital overlap
Orbital overlap was an idea first introduced by Linus Pauling to explain the molecular bond angles observed through experimentation and is the basis for the concept of orbital hybridisation. s orbitals are spherical and have no directionality while p orbitals are oriented 90° to one another...
between the filled cyclopropane bent bonds and the empty p-orbital.
External links
- Press Release The 1994 Nobel Prize in Chemistry". Nobelprize.org.9 Jun 2010