Triphenylmethanol
Encyclopedia
Triphenylmethanol is an organic compound
. It is a white crystalline solid that is insoluble in water and petroleum ether
, but well soluble in ethanol
, diethyl ether
, and benzene
. In strongly acidic solutions, it produces an intensely yellow color, due to the formation of a stable "trityl" carbocation
.
, while the C-O bond length is approximately 1.42 Å
.
It reacts with acetyl chloride to give triphenylmethyl chloride:
s, resonance offers no stabilization of the conjugate base due to its being bonded to a saturated carbon atom. Stabilization of the anion by solvation forces is largely ineffective due to the three phenyl groups.
On the other hand, the basicity of triphenylmethanol is enhanced due to the formation of a stable carbocation upon breaking of the C-O bond. In a highly acid solution, the protonated form of triphenylmethanol will lose the equivalent of a water molecule to form the highly stable triphenylmethylium carbocation.
or benzophenone
and bromobenzene is a common laboratory experiment for teaching the Grignard reaction
. An alternative starting material is diethyl carbonate
.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
. It is a white crystalline solid that is insoluble in water and petroleum ether
Petroleum ether
Petroleum ether, also known as benzine, VM&P Naphtha, Petroleum Naphtha, Naphtha ASTM, Petroleum Spirits, X4 or Ligroin, is a group of various volatile, highly flammable, liquid hydrocarbon mixtures used chiefly as nonpolar solvents...
, but well soluble in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, diethyl ether
Diethyl ether
Diethyl ether, also known as ethyl ether, simply ether, or ethoxyethane, is an organic compound in the ether class with the formula . It is a colorless, highly volatile flammable liquid with a characteristic odor...
, and benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
. In strongly acidic solutions, it produces an intensely yellow color, due to the formation of a stable "trityl" carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
.
Structure and Properties
Triphenylmethanol contains three phenyl rings and a hydroxyl group bound to a central tetrahedral carbon atom. All three C-Ph bonds are typical of sp3-sp2 carbon-carbon bonds with lengths of approximately 1.47 ÅÅngström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
, while the C-O bond length is approximately 1.42 Å
Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
.
It reacts with acetyl chloride to give triphenylmethyl chloride:
- Ph3COH + MeCOCl → Ph3CCl + MeCO2H
Acid-base properties
As a derivative of methanol, triphenylmethanol is expected to have a pKa in the range of 16-19. Typical of alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, resonance offers no stabilization of the conjugate base due to its being bonded to a saturated carbon atom. Stabilization of the anion by solvation forces is largely ineffective due to the three phenyl groups.
On the other hand, the basicity of triphenylmethanol is enhanced due to the formation of a stable carbocation upon breaking of the C-O bond. In a highly acid solution, the protonated form of triphenylmethanol will lose the equivalent of a water molecule to form the highly stable triphenylmethylium carbocation.
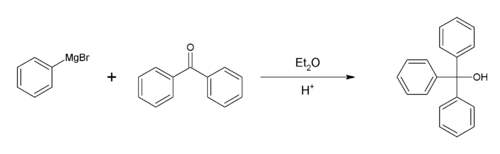
Synthesis
The preparation of triphenylmethanol from methyl benzoateMethyl benzoate
Methyl benzoate is an ester with the chemical formula C6H5COOCH3. It is formed by the condensation of methanol and benzoic acid, in presence of a strong acid such as hydrochloric acid. It is a colorless liquid that is poorly soluble in water, but miscible with organic solvents.-Reactions:Methyl...
or benzophenone
Benzophenone
Benzophenone is the organic compound with the formula 2CO, generally abbreviated Ph2CO. Benzophenone is a widely used building block in organic chemistry, being the parent diarylketone.-Uses:...
and bromobenzene is a common laboratory experiment for teaching the Grignard reaction
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
. An alternative starting material is diethyl carbonate
Diethyl carbonate
Diethyl carbonate is a carbonate ester of carbonic acid and ethanol. At room temperature diethyl carbonate is a clear liquid with a low flash point....
.