
SN1 reaction
Encyclopedia
The SN1 reaction is a substitution reaction
in organic chemistry
. "SN" stands for nucleophilic substitution
and the "1" represents the fact that the rate-determining step
is unimolecular
. The reaction involves a carbocation
intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols. With primary alkyl halides, the alternative SN2 reaction
occurs. Among inorganic chemists, the SN1 reaction is often known as the dissociative mechanism. A reaction mechanism
was first proposed by Christopher Ingold et al. in 1940.
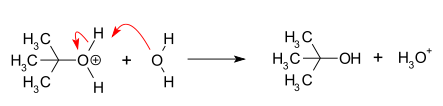
is the hydrolysis
of tert-butyl bromide
with water forming tert-butyl alcohol:
This SN1 reaction takes place in three steps:
stabilization and hyperconjugation
from attached alkyl groups. The Hammond-Leffler postulate suggests that this too will increase the rate of carbocation formation. The SN1 mechanism therefore dominates in reactions at tertiary alkyl centers and is further observed at secondary alkyl centers in the presence of weak nucleophile
s.
An example of a reaction proceeding in a SN1 fashion is the synthesis of 2,5-dichloro-2,5-dimethylhexane from the corresponding diol with concentrated hydrochloric acid
:
As the alpha and beta substitutions increase with respect to leaving groups the reaction is diverted from SN2 to SN1
 However, an excess of one stereoisomer can be observed, as the leaving group can remain in proximity to the carbocation intermediate for a short time and block nucleophilic attack. This stands in contrast to the SN2 mechanism, which is a stereospecific mechanism where stereochemistry is always inverted.
However, an excess of one stereoisomer can be observed, as the leaving group can remain in proximity to the carbocation intermediate for a short time and block nucleophilic attack. This stands in contrast to the SN2 mechanism, which is a stereospecific mechanism where stereochemistry is always inverted.
s and carbocation rearrangement
. If the reaction is performed under warm or hot conditions (which favor an increase in entropy), E1 elimination is likely to predominate, leading to formation of an alkene
. At lower temperatures, SN1 and E1 reactions are competitive reactions and it becomes difficult to favor one over the other. Even if the reaction is performed cold, some alkene may be formed. If an attempt is made to perform an SN1 reaction using a strongly basic nucleophile such as hydroxide
or methoxide
ion, the alkene will again be formed, this time via an E2 elimination. This will be especially true if the reaction is heated. Finally, if the carbocation intermediate can rearrange to a more stable carbocation, it will give a product derived from the more stable carbocation rather than the simple substitution product.
Solvent effects
Since the SN1 reaction involves formation of an unstable carbocation intermediate in the rate-determining step, anything that can facilitate this will speed up the reaction. The normal solvents of choice are both polar
(to stabilize ionic intermediates in general) and protic
(to solvate
the leaving group in particular). Typical polar protic solvents include water and alcohols, which will also act as nucleophiles.
The Y scale correlates solvolysis
reaction rates of any solvent (k) with that of a standard solvent (80% v/v ethanol
/water
) (k0) through

with m a reactant constant (m = 1 for tert-butyl chloride
) and Y a solvent parameter. For example 100% ethanol gives Y = −2.3, 50% ethanol in water Y = +1.65 and 15% concentration Y = +3.2.
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
. "SN" stands for nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
and the "1" represents the fact that the rate-determining step
Rate-determining step
The rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
is unimolecular
Molecularity
Molecularity in chemistry is the number of colliding molecular entities that are involved in a single reaction step. While the order of a reaction is derived experimentally, the molecularity is a theoretical concept and can only be applied to elementary reactions...
. The reaction involves a carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols. With primary alkyl halides, the alternative SN2 reaction
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
occurs. Among inorganic chemists, the SN1 reaction is often known as the dissociative mechanism. A reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
was first proposed by Christopher Ingold et al. in 1940.
Mechanism
An example of a reaction taking place with an SN1 reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
is the hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
of tert-butyl bromide
Tert-Butyl bromide
tert-Butyl bromide is an organic compound with a tert-butyl carbon frame and a bromine substituent. This organobromine compound is used as a raw material in synthetic organic chemistry. The compound is isomeric with 1-bromobutane and 2-bromobutane....
with water forming tert-butyl alcohol:
This SN1 reaction takes place in three steps:
- Formation of a tert-butyl carbocationCarbocationA carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
by separation of a leaving groupLeaving groupIn chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
(a bromideBromideA bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
anion) from the carbon atom: this step is slow and reversibleReversible reactionA reversible reaction is a chemical reaction that results in an equilibrium mixture of reactants and products. For a reaction involving two reactants and two products this can be expressed symbolically as...
.
-
- Nucleophilic attackNucleophileA nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
: the carbocation reacts with the nucleophile. If the nucleophileNucleophileA nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
is a neutral molecule (i.e. a solventSolventA solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
) a third step is required to complete the reaction. When the solvent is water, the intermediate is an oxonium ionOxonium ionThe oxonium ion in chemistry is any oxygen cation with three bonds. The simplest oxonium ion is the hydronium ion H3O+. Another oxonium ion frequently encountered in organic chemistry is obtained by protonation or alkylation of a carbonyl group e.g...
. This reaction step is fast.
- Nucleophilic attack

- DeprotonationDeprotonationDeprotonation is the removal of a proton from a molecule, forming the conjugate base.The relative ability of a molecule to give up a proton is measured by its pKa value. A low pKa value indicates that the compound is acidic and will easily give up its proton to a base...
: Removal of a proton on the protonatedProtonationIn chemistry, protonation is the addition of a proton to an atom, molecule, or ion. Some classic examples include*the protonation of water by sulfuric acid:*the protonation of isobutene in the formation of a carbocation:2C=CH2 + HBF4 → 3C+ + BF4−*the protonation of ammonia in the...
nucleophile by water acting as a base forming the alcoholAlcoholIn chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
and a hydronium ion. This reaction step is fast.
- Deprotonation

Scope of the reaction
The SN1 mechanism tends to dominate when the central carbon atom is surrounded by bulky groups because such groups sterically hinder the SN2 reaction. Additionally, bulky substituents on the central carbon increase the rate of carbocation formation because of the relief of steric strain that occurs. The resultant carbocation is also stabilized by both inductiveInductive effect
In chemistry and physics, the inductive effect is an experimentally observable effect of the transmission of charge through a chain of atoms in a molecule by electrostatic induction...
stabilization and hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
from attached alkyl groups. The Hammond-Leffler postulate suggests that this too will increase the rate of carbocation formation. The SN1 mechanism therefore dominates in reactions at tertiary alkyl centers and is further observed at secondary alkyl centers in the presence of weak nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
s.
An example of a reaction proceeding in a SN1 fashion is the synthesis of 2,5-dichloro-2,5-dimethylhexane from the corresponding diol with concentrated hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
:
As the alpha and beta substitutions increase with respect to leaving groups the reaction is diverted from SN2 to SN1
Stereochemistry
The carbocation intermediate formed in the reaction's rate limiting step is an sp2 hybridized carbon with trigonal planar molecular geometry. This allows two different avenues for the nucleophilic attack, one on either side of the planar molecule. If neither avenue is preferentially favored, these two avenues occur equally, yielding a racemic mix of enantiomers if the reaction takes place at a stereocenter. This is illustrated below in the SN1 reaction of S-3-chloro-3-methylhexane with an iodide ion, which yields a racemic mixture of 3-iodo-3-methylhexane:
Side reactions
Two common side reactions are elimination reactionElimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
s and carbocation rearrangement
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
. If the reaction is performed under warm or hot conditions (which favor an increase in entropy), E1 elimination is likely to predominate, leading to formation of an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
. At lower temperatures, SN1 and E1 reactions are competitive reactions and it becomes difficult to favor one over the other. Even if the reaction is performed cold, some alkene may be formed. If an attempt is made to perform an SN1 reaction using a strongly basic nucleophile such as hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
or methoxide
Methoxide
Methoxides are organic salts and the simplest alkoxides. Sodium methoxide and potassium hydroxide have widespread use, though other variants such as lithium hydroxide, rubidium methoxide, caesium methoxide, and francium methoxide exist as well.- Methoxide ion :In organic chemistry, the methoxide...
ion, the alkene will again be formed, this time via an E2 elimination. This will be especially true if the reaction is heated. Finally, if the carbocation intermediate can rearrange to a more stable carbocation, it will give a product derived from the more stable carbocation rather than the simple substitution product.
Solvent effectsSolvent effectsIn chemistry, Solvent effects is the group of effects that a solvent has on chemical reactivity. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic and kinetic control over a chemical reaction.-Effects on...
Since the SN1 reaction involves formation of an unstable carbocation intermediate in the rate-determining step, anything that can facilitate this will speed up the reaction. The normal solvents of choice are both polarChemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
(to stabilize ionic intermediates in general) and protic
Protic solvent
In chemistry a protic solvent is a solvent that has a hydrogen atom bound to an oxygen or a nitrogen . In general terms, any molecular solvent that contains dissociable H+ is called a protic solvent. The molecules of such solvents can donate an H+...
(to solvate
Solvation
Solvation, also sometimes called dissolution, is the process of attraction and association of molecules of a solvent with molecules or ions of a solute...
the leaving group in particular). Typical polar protic solvents include water and alcohols, which will also act as nucleophiles.
The Y scale correlates solvolysis
Solvolysis
Solvolysis is a special type of nucleophilic substitution or elimination where the nucleophile is a solvent molecule. For certain nucleophiles, there are specific terms for the type of solvolysis reaction...
reaction rates of any solvent (k) with that of a standard solvent (80% v/v ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
/water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
) (k0) through

with m a reactant constant (m = 1 for tert-butyl chloride
Tert-Butyl chloride
tert-Butyl chloride is a colorless, liquid organic compound at room temperature. It is sparingly soluble in water, with a tendency to undergo spontaneous solvolysis when dissolved into it...
) and Y a solvent parameter. For example 100% ethanol gives Y = −2.3, 50% ethanol in water Y = +1.65 and 15% concentration Y = +3.2.
See also
- Nucleophilic acyl substitutionNucleophilic acyl substitutionNucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
- Neighbouring group participationNeighbouring group participationNeighbouring group participation or NGP in organic chemistry has been defined by IUPAC as the interaction of a reaction centre with a lone pair of electrons in an atom or the electrons present in a sigma bond or pi bond . When NGP is in operation it is normal for the reaction rate to be increased...
- Arrow pushingArrow pushingArrow pushing or electron pushing is a technique used to describe the progression of organic chemistry reaction mechanisms. In using arrow pushing, "curved arrows" or "curly arrows" are superimposed over the structural formulae of reactants in a chemical equation to show the reaction mechanism...
Further reading
- Electrophilic Bimolecular Substitution as an Alternative to Nucleophilic Monomolecular Substitution in Inorganic and Organic Chemistry / N.S.Imyanitov. J. Gen. Chem. USSR (Engl. Transl.) 1990; 60 (3); 417-419.
- Unimolecular Nucleophilic Substitution does not Exist! / N.S.Imyanitov. SciTecLibrary
External links
- Diagrams: Frostburg State UniversityFrostburg State UniversityFrostburg State University is a four-year university located on a campus in Frostburg, Maryland, in Western Maryland, and is part of the University System of Maryland. FSU is accredited by the Middle States Commission on Higher Education.-History:...
- Exercise: the University of Maine
- Study Organic Chemistry, Resources for Success in Organic Chemistry

