
Solvation
Encyclopedia
Dissolution (chemistry)
Dissolution is the process by which a solid, liquid or gas forms a solution in a solvent. In solids this can be explained as the breakdown of the crystal lattice into individual ions, atoms or molecules and their transport into the solvent. For liquids and gases, the molecules must be compatible...
, is the process of attraction and association of molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s of a solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
with molecules or ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s of a solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
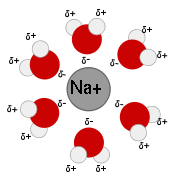
. As ions dissolve in a solvent they spread out and become surrounded by solvent molecules.
Distinction between solvation, dissolution and solubility
By an IUPAC definition, solvation is an interaction of a solute with the solvent, which leads to stabilization of the solute species in the solution. One may also refer to the solvated state, whereby an ion in a solution is complexedComplex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
by solvent molecules. The concept of the solvation interaction can also be applied to an insoluble material, for example, solvation of functional groups on a surface of ion-exchange resin.
Solvation is, in concept, distinct from dissolution
Dissolution (chemistry)
Dissolution is the process by which a solid, liquid or gas forms a solution in a solvent. In solids this can be explained as the breakdown of the crystal lattice into individual ions, atoms or molecules and their transport into the solvent. For liquids and gases, the molecules must be compatible...
and solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
. Dissolution is a kinetic
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
process, and is quantified by its rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
. Solubility
Solubility
Solubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
quantifies the dynamic equilibrium
Dynamic equilibrium
A dynamic equilibrium exists once a reversible reaction ceases to change its ratio of reactants/products, but substances move between the chemicals at an equal rate, meaning there is no net change. It is a particular example of a system in a steady state...
state achieved when the rate of dissolution equals the rate of precipitation
Precipitation (chemistry)
Precipitation is the formation of a solid in a solution or inside anothersolid during a chemical reaction or by diffusion in a solid. When the reaction occurs in a liquid, the solid formed is called the precipitate, or when compacted by a centrifuge, a pellet. The liquid remaining above the solid...
.
The consideration of the units makes the distinction clearer. Complexation can be described by coordination number
Coordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
and the complex stability constants. The typical unit for dissolution rate is mol/s. The unit for solubility can be mol/kg.
Liquefaction accompanied by an irreversible chemical change is also distinct from solvation. For example, zinc cannot be solvated by hydrochloric acid, but it can be converted into the soluble salt zinc chloride by a chemical reaction.
Solvents and intermolecular interactions
PolarChemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
solvents are those with a molecular structure that contains dipoles
Bond dipole moment
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d....
. Such compounds are often found to have a high dielectric constant
Dielectric constant
The relative permittivity of a material under given conditions reflects the extent to which it concentrates electrostatic lines of flux. In technical terms, it is the ratio of the amount of electrical energy stored in a material by an applied voltage, relative to that stored in a vacuum...
. The polar molecules of these solvents can solvate ions because they can orient the appropriate partially-charged portion of the molecule towards the ion in response to electrostatic attraction. This stabilizes the system and creates a solvation shell
Solvation shell
A Solvation shell is a shell of any chemical species acting as a solvent, surrounding a solute species. When the solvent is water it is often referred to as a hydration shell or hydration sphere....
(or hydration shell in the case of water). Water is the most common and well-studied polar solvent, but others exist, such as acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
, dimethyl sulfoxide
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
, methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, propylene carbonate
Propylene carbonate
Propylene carbonate is an organic compound, a cyclic carbonate of propylene glycol. This colorless and odorless liquid is useful as a polar, aprotic solvent...
, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
. These solvents can be used to dissolve inorganic compounds such as salts.
Solvation involves different types of intermolecular interactions: hydrogen bonding, ion-dipole, and dipole-dipole attractions or van der Waals force
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
s. The hydrogen bonding, ion-dipole, and dipole-dipole interactions occur only in polar solvents. Ion-ion interactions occur only in ionic solvents. The solvation process will be thermodynamically favored only if the overall Gibbs energy of the solution is decreased, compared to the Gibbs energy of the separated solvent and solid (or gas or liquid). This means that the change in enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
minus the change in entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
(multiplied by the absolute temperature) is a negative value, or that the Gibbs free energy of the system decreases.
The conductivity
Conductivity (electrolytic)
The conductivity of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter ....
of a solution depends on the solvation of its ions.
Thermodynamic considerations
For solvation to occur, energyEnergy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
is required to release individual ions from the crystal lattices in which they are present. This is necessary to break the attractions the ions have with each other and is equal to the solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
's lattice free energy
Thermodynamic free energy
The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of energy that cannot be used to...
(the energy released at the formation of the lattice as the ions bonded with each other). The energy for this comes from the energy released when ions of the lattice associate with molecules of the solvent. Energy released in this form is called the free energy of solvation.
The enthalpy of solution is the solution enthalpy minus the enthalpy of the separate systems, whereas the entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
is the corresponding difference in entropy. Most gases have a negative enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
of solution. A negative enthalpy of solution means that the solute is less soluble at high temperatures.
Although early thinking was that a higher ratio of a cation's ion charge to the size, or the charge density, resulted in more solvation, this does not stand up to scrutiny for ions like iron(III) or lanthanide
Lanthanide
The lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
s and actinide
Actinide
The actinide or actinoid series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium.The actinide series derives its name from the group 3 element actinium...
s, which are readily hydrolyzed to form insoluble (hydrous) oxides. As solids, these are, it is apparent, not solvated.
Enthalpy of solvation can help explain why solvation occurs with some ionic lattices but not with others. The difference in energy between that which is necessary to release an ion from its lattice and the energy given off when it combines with a solvent molecule is called the enthalpy change of solution
Enthalpy change of solution
The enthalpy of solution, enthalpy of dissolution, or heat of solution is the enthalpy change associated with the dissolution of a substance in a solvent at constant pressure resulting in infinite dilution....
. A negative value for the enthalpy change of solution corresponds to an ion that is likely to dissolve, whereas a high positive value means that solvation will not occur. It is possible that an ion will dissolve even if it has a positive enthalpy value. The extra energy required comes from the increase in entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
that results when the ion dissolves. The introduction of entropy makes it harder to determine by calculation alone whether a substance will dissolve or not. A quantitative measure for solvation power of solvents is given by donor number
Donor number
In chemistry a donor number or DN is a qualitative measure of Lewis basicity. A donor number is defined as the negative enthalpy value for the 1:1 adduct formation between a Lewis base and the standard Lewis acid SbCl5 , in dilute solution in the noncoordinating solvent 1,2-dichloroethane with a...
s.
In general, thermodynamic analysis of solutions is done by modeling them as reactions. For example; if you add sodium chloride(s) to water, the salt will dissociate into the ions sodium(+aq) and chloride(-aq). The equilibrium constant for this dissociation can be predicted by the change in Gibb's free energy of this reaction.
See also
- Complex (chemistry)Complex (chemistry)In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
- SaturationSaturation (chemistry)In chemistry, saturation has six different meanings, all based on reaching a maximum capacity...
- SolubilitySolubilitySolubility is the property of a solid, liquid, or gaseous chemical substance called solute to dissolve in a solid, liquid, or gaseous solvent to form a homogeneous solution of the solute in the solvent. The solubility of a substance fundamentally depends on the used solvent as well as on...
- Solubility equilibriumSolubility equilibriumSolubility equilibrium is a type of dynamic equilibrium. It exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation or with chemical reaction with another constituent of the solvent, such as...
- SoluteSolutionIn chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
- SolutionSolutionIn chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
- SolventSolventA solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
- SupersaturationSupersaturationThe term supersaturation refers to a solution that contains more of the dissolved material than could be dissolved by the solvent under normal circumstances...
- Ideal solutionIdeal solutionIn chemistry, an ideal solution or ideal mixture is a solution with thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of solution is zero as is the volume change on mixing; the closer to zero the enthalpy of solution is, the more "ideal" the behavior of the...

