
Halogen
Encyclopedia
| Group | 17 | |
|---|---|---|
| Period | 2 Period 2 element A period 2 element is one of the chemical elements in the second row of the periodic table. The periodic table is laid out in rows to illustrate recurring trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to... |
|
| 3 Period 3 element A period 3 element is one of the chemical elements in the third row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical... |
||
| 4 Period 4 element A period 4 element is one of the chemical elements in the fourth row of the periodic table of the elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour... |
||
| 5 Period 5 element A period 5 element is one of the chemical elements in the fifth row of the periodic table of the elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour... |
||
| 6 Period 6 element A period 6 element is one of the chemical elements in the sixth row of the periodic table of the elements, including the lanthanides... |
||
| 7 Period 7 element A period 7 element is one of the chemical elements in the seventh row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical... |
The halogens or halogen elements are a series of nonmetal
Nonmetal
Nonmetal, or non-metal, is a term used in chemistry when classifying the chemical elements. On the basis of their general physical and chemical properties, every element in the periodic table can be termed either a metal or a nonmetal...
elements
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
from Group 17
Periodic table group
In chemistry, a group is a vertical column in the periodic table of the chemical elements. There are 18 groups in the standard periodic table, including the d-block elements, but excluding the f-block elements....
IUPAC Style
International Union of Pure and Applied Chemistry
The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in individual countries. It is a member of the International Council for Science . The international headquarters of IUPAC is located in Zürich,...
(formerly: VII, VIIA) of the periodic table
Periodic table
The periodic table of the chemical elements is a tabular display of the 118 known chemical elements organized by selected properties of their atomic structures. Elements are presented by increasing atomic number, the number of protons in an atom's atomic nucleus...
, comprising fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
(F), chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
(Cl), bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
(Br), iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
(I), and astatine
Astatine
Astatine is a radioactive chemical element with the symbol At and atomic number 85. It occurs on the Earth only as the result of decay of heavier elements, and decays away rapidly, so much less is known about this element than its upper neighbors in the periodic table...
(At). The artificially created element 117, provisionally referred to by the systematic name ununseptium
Ununseptium
Ununseptium is the temporary name of a superheavy artificial chemical element with temporary symbol Uus and atomic number 117. Six atoms were detected by a joint Russia–US collaboration at Dubna, Moscow Oblast, Russia, in 2009–10...
, may also be a halogen.
The group of halogens is the only periodic table group which contains elements in all three familiar states of matter at standard temperature and pressure
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
.
Abundance
Owing to their high reactivity, the halogens are found in the environment only in compoundsChemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
or as ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s. Halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
ions and oxoanions
Oxyanion
An oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
such as iodate
Iodate
An iodate is a conjugate base of iodic acid. In the iodate anion, iodine is bonded to three oxygen atoms and the molecular formula is IO3−. The molecular geometry of iodate is trigonal pyramidal....
(IO3−) can be found in many minerals and in seawater. Halogenated organic compounds can also be found as natural products in living organisms. In their elemental forms, the halogens exist as diatomic molecules, but these only have a fleeting existence in nature and are much more common in the laboratory and in industry. At room temperature and pressure, fluorine and chlorine are gases, bromine is a liquid and iodine and astatine are solids; Group 17 is therefore the only periodic table group exhibiting all three states of matter
State of matter
States of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
at room temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
.
Etymology
In 1842 the SwedishSweden
Sweden , officially the Kingdom of Sweden , is a Nordic country on the Scandinavian Peninsula in Northern Europe. Sweden borders with Norway and Finland and is connected to Denmark by a bridge-tunnel across the Öresund....
chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
Baron Jöns Jakob Berzelius
Jöns Jakob Berzelius
Jöns Jacob Berzelius was a Swedish chemist. He worked out the modern technique of chemical formula notation, and is together with John Dalton, Antoine Lavoisier, and Robert Boyle considered a father of modern chemistry...
proposed the term "halogen" – ἅλς (háls), "salt" or "sea", and γεν- (gen-), from γίγνομαι (gígnomai), "come to be" – for the four elements (fluorine, chlorine, bromine, and iodine) that produce a sea-salt-like substance when they form a compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with a metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
. Earlier, in 1811, the word "halogen" had been proposed as a name for the newly discovered element chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, but Davy's proposed term for this element eventually won out.
Properties
Like other groups, the candidates of this family show patterns in its electron configurationElectron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
, especially the outermost shells resulting in trends in chemical behavior:
| Z Atomic number In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element... | Element Chemical element A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements... | No. of electrons/shell Electron shell An electron shell may be thought of as an orbit followed by electrons around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" , followed by the "2 shell" , then the "3 shell" , and so on further and further from the nucleus. The shell letters K,L,M,..... |
|---|---|---|
| 9 | fluorine | 2, 7 |
| 17 | chlorine | 2, 8, 7 |
| 35 | bromine | 2, 8, 18, 7 |
| 53 | iodine | 2, 8, 18, 18, 7 |
| 85 | astatine | 2, 8, 18, 32, 18, 7 |
The halogens show a series of trends when moving down the group—for instance, decreasing electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
and reactivity, and increasing melting
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
and boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
.
| Halogen | Standard Atomic Weight (u) | Melting Point (K Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... ) | Boiling Point (K Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... ) | Electronegativity (Pauling) |
| Fluorine Fluorine Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic... | 18.998 | 53.53 | 85.03 | 3.98 |
| Chlorine Chlorine Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine... | 35.453 | 171.60 | 239.11 | 3.16 |
| Bromine Bromine Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826... | 79.904 | 265.80 | 332.00 | 2.96 |
| Iodine Iodine Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.... | 126.904 | 386.85 | 457.40 | 2.66 |
| Astatine Astatine Astatine is a radioactive chemical element with the symbol At and atomic number 85. It occurs on the Earth only as the result of decay of heavier elements, and decays away rapidly, so much less is known about this element than its upper neighbors in the periodic table... | (210) | 575.00 | 610 (?) | 2.20 |
Diatomic halogen molecules
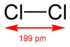
| halogen | molecule | structure | model | d(X−X) / pm (gas phase) |
d(X−X) / pm (solid phase) |
|---|---|---|---|---|---|
| Fluorine Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic... |
|
 |
 |
|
|
| Chlorine Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine... |
|
 |
 |
|
|
| Bromine Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826... |
|
 |
 |
|
|
| Iodine Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor.... |
|
 |
 |
|
|
The elements become less reactive and have higher melting points as the atomic number increases.
Reactivity
Halogens are highly reactive, and as such can be harmful or lethal to biological organismsOrganism
In biology, an organism is any contiguous living system . In at least some form, all organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homoeostasis as a stable whole.An organism may either be unicellular or, as in the case of humans, comprise...
in sufficient quantities. This high reactivity is due to the atoms being highly electronegative
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
due to their high effective nuclear charge
Effective nuclear charge
The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom. The term "effective" is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge by the repelling effect...
. They can gain an electron by reacting with atoms of other elements. Fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
is one of the most reactive elements in existence, attacking otherwise inert materials such as glass, and forming compounds with the heavier noble gases. It is a corrosive and highly toxic gas. The reactivity of fluorine is such that if used or stored in laboratory glassware, it can react with glass in the presence of small amounts of water to form silicon tetrafluoride
Silicon tetrafluoride
Silicon tetrafluoride or Tetrafluorosilane is the chemical compound with the formula SiF4. This tetrahedral molecule is notable for having a remarkably narrow liquid range...
(SiF4). Thus fluorine must be handled with substances such as Teflon
Polytetrafluoroethylene
Polytetrafluoroethylene is a synthetic fluoropolymer of tetrafluoroethylene that finds numerous applications. PTFE is most well known by the DuPont brand name Teflon....
(which is itself an organofluorine
Organofluorine
Organofluorine compounds are organic chemical compounds that contain carbon and fluorine bonded in the polarized and remarkably strong carbon–fluorine bond. Organofluorine compounds are diverse, they can be fluorocarbons, perfluorinated, or biologically synthesized mono-fluorinated compounds, among...
compound), extremely dry glass, or metals such as copper or steel which form a protective layer of fluoride on their surface.
The high reactivity of fluorine means that once it does react with something, it bonds with it so strongly that the resulting molecule is very inert and non-reactive to anything else. For example, Teflon is fluorine bonded with carbon.
Both chlorine and bromine are used as disinfectants for drinking water, swimming pools, fresh wounds, spas, dishes, and surfaces. They kill bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
and other potentially harmful microorganisms through a process known as sterilization
Sterilization (microbiology)
Sterilization is a term referring to any process that eliminates or kills all forms of microbial life, including transmissible agents present on a surface, contained in a fluid, in medication, or in a compound such as biological culture media...
. Their reactivity is also put to use in bleaching. Sodium hypochlorite, which is produced from chlorine, is the active ingredient of most fabric bleaches and chlorine-derived bleaches are used in the production of some paper
Paper
Paper is a thin material mainly used for writing upon, printing upon, drawing or for packaging. It is produced by pressing together moist fibers, typically cellulose pulp derived from wood, rags or grasses, and drying them into flexible sheets....
products. Chlorine also reacts with sodium to create sodium chloride, which is another name for table salt.
Hydrogen halides
The halogens all form binary compounds with hydrogen known as the hydrogen halideHydrogen halide
Hydrogen halides are acids resulting from the chemical reaction of hydrogen with one of the halogen elements , which are found in Group 17 of the periodic table. Astatine is not included in the list because it is very rare, unstable and not found as the acid in substantial quantities...
s (HF
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
, HCl
Hydrogen chloride
The compound hydrogen chloride has the formula HCl. At room temperature, it is a colorless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric humidity. Hydrogen chloride gas and hydrochloric acid are important in technology and industry...
, HBr
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
, HI
Hydrogen iodide
Hydrogen iodide is a diatomic molecule. Aqueous solutions of HI are known as iohydroic acid or hydroiodic acid, a strong acid. Gas and aqueous solution are interconvertible...
, and HAt
Hydrogen astatide
Hydrogen astatide, also known as astatane, or astidohydrogen is a chemical compound with the chemical formula HAt, consisting of an astatine atom covalently bonded to a hydrogen atom....
), a series of particularly strong acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s. When in aqueous solution, the hydrogen halides are known as hydrohalic acids. HAt, or "hydroastatic acid", should also qualify, but it is not typically included in discussions of hydrohalic acid due to astatine's extreme instability toward alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
.
Interhalogen compounds
The halogens react with each other to form interhalogen compounds. Diatomic interhalogen compounds such as BrF, IClIodine monochloride
Iodine monochloride is an interhalogen compound with the formula ICl. It is a red-brown compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, ICl is highly polar and behaves as a source of I+....
, and ClF
Chlorine monofluoride
Chlorine monofluoride is a volatile interhalogen compound with the chemical formula ClF. It is a colourless gas at room temperature and is stable even at high temperatures. When cooled to −100 °C, ClF condenses as a pale yellow liquid...
bear resemblance to the pure halogens in some respects. The properties and behaviour of a diatomic interhalogen compound tend to be intermediate between those of its parent halogens. Some properties, however, are found in neither parent halogen. For example, Cl2 and I2 are soluble in CCl4
Carbon tetrachloride
Carbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
, but ICl is not since it is a polar molecule
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
due to the relatively large electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
difference between I and Cl.
Organohalogen compounds
Many synthetic organic compounds such as plasticPlastic
A plastic material is any of a wide range of synthetic or semi-synthetic organic solids used in the manufacture of industrial products. Plastics are typically polymers of high molecular mass, and may contain other substances to improve performance and/or reduce production costs...
polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s, and a few natural ones, contain halogen atoms; these are known as halogenated compounds or organic halides. Chlorine is by far the most abundant of the halogens, and the only one needed in relatively large amounts (as chloride ions) by humans. For example, chloride ions play a key role in brain
Brain
The brain is the center of the nervous system in all vertebrate and most invertebrate animals—only a few primitive invertebrates such as sponges, jellyfish, sea squirts and starfishes do not have one. It is located in the head, usually close to primary sensory apparatus such as vision, hearing,...
function by mediating the action of the inhibitory transmitter GABA
Gamma-aminobutyric acid
γ-Aminobutyric acid is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays a role in regulating neuronal excitability throughout the nervous system...
and are also used by the body to produce stomach acid. Iodine is needed in trace amounts for the production of thyroid
Thyroid
The thyroid gland or simply, the thyroid , in vertebrate anatomy, is one of the largest endocrine glands. The thyroid gland is found in the neck, below the thyroid cartilage...
hormones such as thyroxine
Thyroxine
Thyroxine, or 3,5,3',5'-tetraiodothyronine , a form of thyroid hormones, is the major hormone secreted by the follicular cells of the thyroid gland.-Synthesis and regulation:...
. On the other hand, neither fluorine nor bromine are believed to be essential for humans.
Polyhalogenated compounds
Polyhalogenated compoundPolyhalogenated compound
Polyhalogenated compounds are any compounds with multiple substitutions of halogens. They are of particular interest and importance because halogens generally are highly reactive and also bioaccumulate in humans, and comprise a superset of which has many toxic and carcinogenic industrial...
s are industrially created compounds substituted with multiple halogens. Many of them are very toxic and bioaccumulate in humans, and have a very wide application range. They include the much maligned PCB
Polychlorinated biphenyl
Polychlorinated biphenyls are a class of organic compounds with 2 to 10 chlorine atoms attached to biphenyl, which is a molecule composed of two benzene rings. The chemical formula for PCBs is C12H10-xClx...
s, PBDE
PBDE
Polybrominated diphenyl ethers or PBDE, are organobromine compounds that are used as flame retardants. Like other brominated flame retardants, PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics,...
s, and PFC
Perfluorinated compounds
A perfluorinated compound is an organofluorine compound with all hydrogens replaced by fluorine on a carbon chain—but the molecule also contains at least one different atom or functional group. Thus, PFCs have properties similar to fluorocarbons as they are fluorocarbon derivatives...
s as well as numerous other compounds.
Drug discovery
In drug discoveryDrug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which drugs are discovered or designed.In the past most drugs have been discovered either by identifying the active ingredient from traditional remedies or by serendipitous discovery...
, the incorporation of halogen atoms into a lead drug candidate results in analogues that are usually more lipophilic
Lipophilic
Lipophilicity, , refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. These non-polar solvents are themselves lipophilic — the axiom that like dissolves like generally holds true...
and less water soluble. Consequently, halogen atoms are used to improve penetration through lipid membranes and tissues. It follows that there is a tendency for some halogenated drugs to accumulate in adipose tissue
Adipose tissue
In histology, adipose tissue or body fat or fat depot or just fat is loose connective tissue composed of adipocytes. It is technically composed of roughly only 80% fat; fat in its solitary state exists in the liver and muscles. Adipose tissue is derived from lipoblasts...
.
The chemical reactivity of halogen atoms depends on both their point of attachment to the lead and the nature of the halogen. Aromatic halogen groups are far less reactive than aliphatic halogen groups, which can exhibit considerable chemical reactivity. For aliphatic carbon-halogen bonds the C-F bond is the strongest and usually less chemically reactive than aliphatic C-H bonds. The other aliphatic-halogen bonds are weaker, their reactivity increasing down the periodic table. They are usually more chemically reactive than aliphatic C-H bonds. Consequently, the most common halogen substitutions are the less reactive aromatic fluorine and chlorine groups.
Reactivity with water
Fluorine reacts vigorously with water to produce oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(O2) and hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
(HF):
- 2 F2(g) + 2 H2O(l) → O2(g) + 4 HF(aq)
Chlorine has minimal solubility of 0.7g Cl2 per kg of water at ambient temperature (21oC). Dissolved chlorine reacts to form hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
(HCl) and hypochlorous acid
Hypochlorous acid
Hypochlorous acid is a weak acid with the chemical formula HClO. It forms when chlorine dissolves in water. It cannot be isolated in pure form due to rapid equilibration with its precursor...
, a solution that can be used as a disinfectant or bleach
Bleach
Bleach refers to a number of chemicals that remove color, whiten, or disinfect, often via oxidation. Common chemical bleaches include household chlorine bleach , lye, oxygen bleach , and bleaching powder...
:
- Cl2(g) + H2O(l) → HCl(aq) + HClO(aq)
Bromine has a solubility of 3.41 g per 100 g of water, but it slowly reacts to form hydrogen bromide
Hydrogen bromide
Hydrogen bromide is the diatomic molecule HBr. HBr is a gas at standard conditions. Hydrobromic acid forms upon dissolving HBr in water. Conversely, HBr can be liberated from hydrobromic acid solutions with the addition of a dehydration agent, but not by distillation. Hydrogen bromide and...
(HBr) and hypobromous acid
Hypobromous acid
Hypobromous acid is a weak, unstable acid with chemical formula HBrO. It is also called bromic acid, bromanol or hydroxidobromine. It occurs only in solution and has chemical and physical properties that are very similar to those of hypochlorous acid.In aqueous solution, hypobromous acid partially...
(HBrO):
- Br2(g) + H2O(l) → HBr(aq) + HBrO(aq)
Iodine, however, is minimally soluble in water (0.03 g/100 g water @ 20 °C) and does not react with it. However, iodine will form an aqueous solution in the presence of iodide ion, such as by addition of potassium iodide
Potassium iodide
Potassium iodide is an inorganic compound with the chemical formula KI. This white salt is the most commercially significant iodide compound, with approximately 37,000 tons produced in 1985. It is less hygroscopic than sodium iodide, making it easier to work with...
(KI), because the triiodide
Triiodide
In chemistry, triiodide can have several meanings. Triiodide primarily refers to the triiodide ion, I3−, a polyatomic anion composed of three iodine atoms. For some chemical compounds, triiodide indicates a salt of the named cation with the triiodide anion. Examples include sodium triiodide, ...
ion is formed.
Further reading
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997.
| Halogens | Atomic numbers in are gases | Atomic numbers in are liquids | Atomic numbers in are solids |
|---|---|---|---|
| Solid borders indicate primordial elements (older than the Earth Earth Earth is the third planet from the Sun, and the densest and fifth-largest of the eight planets in the Solar System. It is also the largest of the Solar System's four terrestrial planets... ) |
Dashed borders indicate radioactive Radioactive decay Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom... natural elements |
Dotted borders indicate radioactive synthetic element Synthetic element In chemistry, a synthetic element is a chemical element that is too unstable to occur naturally on Earth, and therefore has to be created artificially. So far 30 synthetic elements have been discovered—that is, synthesized... s |
No borders indicates undiscovered elements |

