Tosylhydrazone
Encyclopedia
A tosylhydrazone in organic chemistry
is a functional group
with the general structure RR'C=NH-Ts where Ts is a tosyl
group. Organic compounds having this functional group can be accessed by reaction of an aldehyde
or ketone
with tosylhydrazine.
and tosylhydrazine in ethanol
with hydrochloric acid
catalysis.
is the reverse reaction of formation with regeneration of the carbonyl compound.
In the Shapiro reaction
tosylhydrazones are used as leaving group
in elimination reaction
s. This reaction required a strong base. With sodium as a base the reaction is called the Bamford–Stevens reaction. Tosylhydrazones can be reduced to the corresponding alkanes with reagents such as sodium borohydride
and borane
.
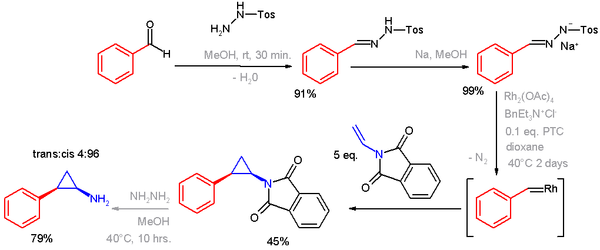
Tosylhydrazone salts can react with metals to form metal carbenes and used in cyclopropanations and epoxidations. An example of a transition metal
-catalyzed cyclopropanation
is a synthesis of tranylcypromine
, in which the sodium salt of benzaldehyde tosylhydrazone is converted to a rhodium
metal carbene through the diazo intermediate.
Tosylhydrazones are also starting materials for certain cross-coupling reactions. In the first report on this reaction type the coupling partners were a tosylhydrazone, an aryl halide with catalyst system dibenzylideneacetone
/ XPhos
. As part of the catalytic cycle
the diazo intermediateformed by decomposition of the tosylhydrazone forms a palladium-carbene complex with the oxidative addition complex of palladium with the aryl halide.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
is a functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
with the general structure RR'C=NH-Ts where Ts is a tosyl
Tosyl
A tosyl group is CH3C6H4SO2. This group is usually derived from the compound 4-toluenesulfonyl chloride, CH3C6H4SO2Cl, which forms esters and amides of toluenesulfonic or tosylic acid...
group. Organic compounds having this functional group can be accessed by reaction of an aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
with tosylhydrazine.
Synthesis
As an example camphor tosylhydrazone is synthesised from camphorCamphor
Camphor is a waxy, white or transparent solid with a strong, aromatic odor. It is a terpenoid with the chemical formula C10H16O. It is found in wood of the camphor laurel , a large evergreen tree found in Asia and also of Dryobalanops aromatica, a giant of the Bornean forests...
and tosylhydrazine in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
with hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
catalysis.
Reactions
HydrolysisHydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
is the reverse reaction of formation with regeneration of the carbonyl compound.
In the Shapiro reaction
Shapiro reaction
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of strong base. The reaction was discovered by Robert H. Shapiro in 1975...
tosylhydrazones are used as leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
in elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
s. This reaction required a strong base. With sodium as a base the reaction is called the Bamford–Stevens reaction. Tosylhydrazones can be reduced to the corresponding alkanes with reagents such as sodium borohydride
Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
and borane
Borane
In chemistry, a borane is a chemical compound of boron and hydrogen. The boranes comprise a large group of compounds with the generic formulae of BxHy. These compounds do not occur in nature. Many of the boranes readily oxidise on contact with air, some violently. The parent member BH3 is called...
.
Tosylhydrazone salts can react with metals to form metal carbenes and used in cyclopropanations and epoxidations. An example of a transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
-catalyzed cyclopropanation
Cyclopropane
Cyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
is a synthesis of tranylcypromine
Tranylcypromine
Tranylcypromine is a drug of the substituted phenethylamine and amphetamine classes which acts as a monoamine oxidase inhibitor —it is a non-selective and irreversible inhibitor of the enzyme monoamine oxidase...
, in which the sodium salt of benzaldehyde tosylhydrazone is converted to a rhodium
Rhodium
Rhodium is a chemical element that is a rare, silvery-white, hard and chemically inert transition metal and a member of the platinum group. It has the chemical symbol Rh and atomic number 45. It is composed of only one isotope, 103Rh. Naturally occurring rhodium is found as the free metal, alloyed...
metal carbene through the diazo intermediate.
Tosylhydrazones are also starting materials for certain cross-coupling reactions. In the first report on this reaction type the coupling partners were a tosylhydrazone, an aryl halide with catalyst system dibenzylideneacetone
Dibenzylideneacetone
Dibenzylideneacetone or dibenzalacetone, often abbreviated dba, is an organic compound with the formula C17H14O. It is a bright-yellow solid insoluble in water, but soluble in ethanol. Dibenzylideneacetone is used as a sunscreen component and as a ligand in organometallic chemistry, for instance in...
/ XPhos
XPhos
XPhos is an organophosphorus compound derived from biphenyl. Its palladium complexes exhibit high activity for Buchwald-Hartwig amination reactions involving aryl chlorides and aryl tosylates. Both palladium and copper complexes of the compound exhibit high activity for the coupling of aryl...
. As part of the catalytic cycle
Catalytic cycle
A catalytic cycle in chemistry is a term for a multistep reaction mechanism that involves a catalyst . The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, materials science, etc. Often such cycles show the conversion of a...
the diazo intermediateformed by decomposition of the tosylhydrazone forms a palladium-carbene complex with the oxidative addition complex of palladium with the aryl halide.