Finkelstein reaction
Encyclopedia
The Finkelstein reaction, named for the German chemist Hans Finkelstein , is an SN2 reaction
that involves the exchange of one halogen
atom for another. Halide exchange is an equilibrium reaction
, but the reaction can be driven to completion by taking advantage of differential solubility of halide salts, or by using a large excess of the halide salt.
The classic Finkelstein reaction involves the conversion of an alkyl chloride or an alkyl bromide to an alkyl iodide by the addition of sodium iodide
in acetone
. Because sodium iodide is soluble in acetone and sodium chloride
and sodium bromide
are not, the equilibrium is shifted by the precipitation of the insoluble salt. For example, bromoethane
can be converted to iodoethane:
Alkyl halides differ greatly in the ease with which they undergo the Finkelstein reaction. The reaction works well for primary (except for neopentyl) halides, and exceptionally well for allyl
, benzyl
, and α-carbonyl halides. Secondary substrates are marginal. Vinyl
, aryl
and tertiary alkyl halides are unreactive. Below some relative rates of reaction (NaI in acetone at 60°):
In modern usage the definition of the reaction has been expanded to include the conversion of alcohol
s to alkyl halides by first converting the alcohol to a sulfonate
ester (tosylates or mesylate
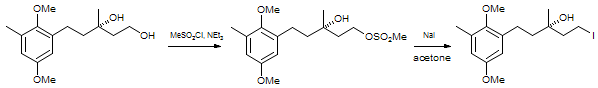
s are usually used), and then performing the substitution. The example below is from a synthesis of Chrysochlamic Acid.
with potassium fluoride
using polar solvents such as DMF and DMSO and high temperatures .
SN2 reaction
The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step...
that involves the exchange of one halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
atom for another. Halide exchange is an equilibrium reaction
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, but the reaction can be driven to completion by taking advantage of differential solubility of halide salts, or by using a large excess of the halide salt.
- R-X + X′− R-X′ + X−
The classic Finkelstein reaction involves the conversion of an alkyl chloride or an alkyl bromide to an alkyl iodide by the addition of sodium iodide
Sodium iodide
Sodium iodide is a white, crystalline salt with chemical formula NaI used in radiation detection, treatment of iodine deficiency, and as a reactant in the Finkelstein reaction.-Uses:Sodium iodide is commonly used to treat and prevent iodine deficiency....
in acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
. Because sodium iodide is soluble in acetone and sodium chloride
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
and sodium bromide
Sodium bromide
Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.-Synthesis, structure, reactions:...
are not, the equilibrium is shifted by the precipitation of the insoluble salt. For example, bromoethane
Bromoethane
Bromoethane, also known as ethyl bromide, is a chemical compound of the haloalkanes group. It is abbreviated by chemists as EtBr. This volatile compound has an ether-like odour.-Preparation:...
can be converted to iodoethane:
- CH3CH2Br (acetone) + NaI (acetone) → CH3CH2I (acetone) + NaBr (s)
Alkyl halides differ greatly in the ease with which they undergo the Finkelstein reaction. The reaction works well for primary (except for neopentyl) halides, and exceptionally well for allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
, benzyl
Benzyl
In organic chemistry, benzyl is the term used to describe the substituent or molecular fragment possessing the structure C6H5CH2-. Benzyl features a benzene ring attached to a CH2 group.-Nomenclature:...
, and α-carbonyl halides. Secondary substrates are marginal. Vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
, aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
and tertiary alkyl halides are unreactive. Below some relative rates of reaction (NaI in acetone at 60°):
| Me-Cl | Bu-Cl | i-Pr-Cl | t-BuCH2-Cl | CH2=CH-CH2-Cl | PhCH2-Cl | EtOC(O)CH2-Cl | MeC(O)CH2-Cl |
|---|---|---|---|---|---|---|---|
| 179 | 1 | 0.0146 | 0.00003 | 64 | 179 | 1600 | 33000 |
In modern usage the definition of the reaction has been expanded to include the conversion of alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s to alkyl halides by first converting the alcohol to a sulfonate
Sulfonate
A sulfonate is a salt or ester of a sulfonic acid. It contains the functional group R-SO2O-.- Sulfonate salts:Anions with the general formula RSO2O− are called sulfonates. They are the conjugate bases of sulfonic acids with formula RSO2OH. As sulfonic acids tend to be strong acids, the...
ester (tosylates or mesylate
Mesylate
In chemistry, a mesylate is any salt or ester of methanesulfonic acid . In salts, the mesylate is present as the CH3SO3− anion. When modifying the International Nonproprietary Name of a pharmaceutical substance containing the group or anion, the correct spelling is mesilate .Mesylate esters are a...
s are usually used), and then performing the substitution. The example below is from a synthesis of Chrysochlamic Acid.
Halex reaction
The halex reaction describes any aryl HALogen EXchange. The chlorine atom in aryl chlorides (with electron-withdrawing substituents) is replaced by fluorineFluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
with potassium fluoride
Potassium fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali metal halide and occurs naturally as the rare mineral carobbiite...
using polar solvents such as DMF and DMSO and high temperatures .