
Rebreather
Encyclopedia
A rebreather is a type of breathing set
that provides a breathing gas
containing oxygen
and recycled exhaled gas. This recycling reduces the volume of breathing gas used, making a rebreather lighter and more compact than an open-circuit breathing set
for the same duration in environments where humans cannot safely breathe from the atmosphere. In the armed forces
it is sometimes called "CCUBA" (Closed Circuit Underwater Breathing Apparatus).
Rebreather technology is used in many environments:
and makes carbon dioxide
. At shallow depths, a person with an open-circuit breathing set typically only uses about a quarter of the oxygen in the air that is breathed in (4%–5% of the inspired volume). The remaining oxygen is exhaled along with nitrogen
and carbon dioxide. As the diver goes deeper, roughly the same quantity of oxygen is used, which represents an increasingly smaller fraction of the compressed air breathed in. Because exhaled air can contain as much as 79% nitrogen (which is not utilized in the body) and 16% (or more) unused oxygen, every exhaled breath from an open-circuit scuba set represents at least 95% wasted, potentially useful gas volume, which has to be replaced from the air supply.
The rebreather recirculates the exhaled gas for re-use and does not discharge it to the atmosphere or water. It absorbs the carbon dioxide, which otherwise would accumulate and cause carbon dioxide poisoning. It removes the carbon dioxide by a process called scrubbing
. The rebreather adds oxygen, to replace the oxygen that was consumed. Thus, the gas in the rebreather's circuit remains breathable and supports life and the diver needs only a fraction of the gas that would be required for an open-circuit system.

) is inert, the diver on open-circuit scuba only uses about 5% of his cylinders' contents.
At depth, the advantage of a rebreather is even more marked. Since the generation of CO2 is directly related to the body's consumption of O2 (about ~99.5% of O2 is converted to CO2 on exhalation), the amount of O2 consumption doesn't change, therefore CO2 generation doesn't change. This means that at depth, the diver is not using any more of the O2 gas supply than when shallower. This is a marked difference from open circuit where the amount of gas consumed increases as depth increases.
s the diver can carry. The economy of gas consumption is also useful when the gas mix being breathed contains expensive gases, such as helium
. In normal use, only oxygen is consumed: small volumes of expensive inert gases are reused during (only) one dive, due to venting of the gas on ascent. For example, a closed circuit rebreather diver effectively doesn't use any of their diluent gas once they've reached the bottom phase of the dive; they could turn off their diluent. On ascent, no diluent is added, however most of that in circuit is lost. A very small amount of trimix would then last for many dives. It is not uncommon for a 3 litre (19 cubic foot) diluent cylinder to last for eight 40 m (131.2 ft) dives.
and underwater photography
to avoid alarming marine animals and thereby get closer to them. This lack of exhale also allows shipwreck divers to enter enclosed areas on sunken ships and avoid slowly filling them with air, which then supports the growth of rust.
The fully closed circuit rebreather is able to minimise the proportion of inert gases in the breathing mix, and therefore minimise the decompression requirements of the diver, by maintaining a specific and relatively high oxygen partial pressure
(ppO2) at all depths. The breathing gas in a rebreather is warmer and more moist than the dry and cold gas from open circuit equipment making it more comfortable to breathe on long dives and causing less dehydration in the diver.
Most modern rebreathers have a system of very sensitive oxygen sensors, which allow the diver to adjust the partial pressure of oxygen. This can offer a dramatic advantage at the end of deeper dives, where a diver can raise the partial pressure of oxygen somewhat at shallower depth, in order to shorten decompression times. Care must be taken that the ppO2 is not set to a level where it can become toxic though. Research has shown that a ppO2 of 1.6 bar is toxic with extended exposure
One major difference between rebreather diving and open-circuit scuba diving is in keeping neutral buoyancy. When an open-circuit scuba diver inhales, a quantity of highly compressed gas from his cylinder is reduced in pressure by a regulator, and enters the lungs at a much higher volume than it occupied in the cylinder. This means that the diver has a tendency to rise slightly with each inhalation, and lower slightly with each exhalation. This does not happen to a rebreather diver, because the diver is circulating a roughly constant volume of gas between his lungs and the breathing bag.
 This is the oldest type of rebreather and was commonly used by navies
This is the oldest type of rebreather and was commonly used by navies
from the early twentieth century. Oxygen rebreathers can be remarkably simple designs, and their invention predates that of open-circuit scuba. The only gas that it supplies is oxygen. As pure oxygen is toxic
when inhaled at pressure, oxygen rebreathers are currently limited to a depth of 6 meters (20 ft); some say 9 meters (30 ft). In the past they have been used deeper (up to 20 meters) but such dives were more risky than what is now considered acceptable. Oxygen rebreathers are also sometimes used when decompressing from a deep open-circuit dive, as breathing pure oxygen makes the nitrogen diffuse out of the blood more rapidly.
The diving pioneer Hans Hass
used Dräger
oxygen rebreathers in the early 1940s.
In some rebreathers, e.g. the Siebe Gorman Salvus
, the oxygen cylinder has two first stages in parallel. One is constant flow; the other is a plain on-off valve called a bypass
; both feed into the same exit pipe which feeds the breathing bag. In the Salvus there is no second stage and the gas is turned on and off at the cylinder. Some simple oxygen rebreathers had no constant-flow valve, but only the bypass, and the diver had to operate the valve at intervals to refill the breathing bag as he used the oxygen.
Oxygen rebreathers are no longer commonly used in diving because of the depth limit imposed by oxygen toxicity. However, they are still the most commonly used for industrial applications on the surface, (SCBA) such as in mines, due to their simplicity and compact size.
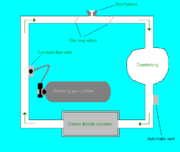 Military and recreational divers use these because they provide better underwater duration than open circuit, have a deeper maximum operating depth
Military and recreational divers use these because they provide better underwater duration than open circuit, have a deeper maximum operating depth
than oxygen rebreathers and are fairly simple and cheap.
Semi-closed circuit equipment generally supplies one breathing gas such as air or nitrox or trimix. The gas is injected into the loop at a constant rate to replenish oxygen consumed from the loop by the diver. Excess gas must be constantly vented from the loop in small volumes to make space for fresh, oxygen-rich gas. As the oxygen in the vented gas cannot be separated from the inert gas, semi-closed circuit is wasteful of oxygen.
The diver must fill the cylinders with gas mix that has a maximum operating depth that is safe for the depth of the dive being planned.
As the amount of oxygen required by the diver increases with work rate, the gas injection rate must be carefully chosen and controlled to prevent unconsciousness
in the diver due to hypoxia
. A higher gas injection rate reduces the likelihood of hypoxia but consumes more gas and wastes more oxygen.
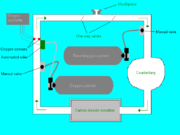 Military, photographic, and recreational divers use these because they allow long dives and produce no bubbles. Closed circuit rebreathers generally supply two breathing gases to the loop: one is pure oxygen and the other is a diluent or diluting gas such as air or trimix.
Military, photographic, and recreational divers use these because they allow long dives and produce no bubbles. Closed circuit rebreathers generally supply two breathing gases to the loop: one is pure oxygen and the other is a diluent or diluting gas such as air or trimix.
The major task of the fully closed circuit rebreather is to control the oxygen concentration, known as the oxygen partial pressure
, in the loop and to warn the diver if it is becoming dangerously low or high. The concentration of oxygen in the loop depends on two factors: depth and the proportion of oxygen in the mix. Too low a concentration of oxygen results in hypoxia leading to unconsciousness and ultimately death
. Too high a concentration of oxygen results in hyperoxia, leading to oxygen toxicity
, a condition causing convulsions which can make the diver lose the mouthpiece when they occur underwater, and can lead to drowning
.
In fully automatic closed-circuit systems, a mechanism injects oxygen into the loop when it detects that the partial pressure of oxygen in the loop has fallen below the required level. Often this mechanism is electrical and relies on oxygen sensitive electro-galvanic fuel cell
s called “ppO2 meters” to measure the concentration of oxygen in the loop.
The diver may be able to manually control the mixture by adding diluent gas or oxygen. Adding diluent can prevent the loop's gas mixture becoming too oxygen rich. Manually adding oxygen is risky as additional small volumes of oxygen in the loop can easily raise the partial pressure of oxygen to dangerous levels.
was designed to be run in this mode or as an ordinary rebreather.
Tests on the IDA71 at the United States Navy Experimental Diving Unit
in Panama City, Florida
showed that the IDA71 could give significantly longer dive time with superoxide in one of the canisters than without.
 If used underwater, the liquid-oxygen tank must be well insulated against heat coming in from the water. As a result, industrial sets of this type may not be suitable for diving, and diving sets of this type may not be suitable for use out of water. The set's liquid oxygen tank must be filled immediately before use. They include these types:
If used underwater, the liquid-oxygen tank must be well insulated against heat coming in from the water. As a result, industrial sets of this type may not be suitable for diving, and diving sets of this type may not be suitable for use out of water. The set's liquid oxygen tank must be filled immediately before use. They include these types:
A cryogenic rebreather called the S-1000 was built around or soon after 1960 by Sub-Marine Systems Corporation. It had a duration of 6 hours and a maximum dive depth of 200 meters sea water. Its ppO2 could be set to anything from 0.2 bar to 2 bar without electronics, by controlling the temperature of the liquid oxygen, thus controlling the equilibrium pressure of oxygen gas above the liquid. The diluent could be either liquid nitrogen
or helium depending on the depth of the dive. The set could freeze out 230 grams of carbon dioxide
per hour from the loop, corresponding to an oxygen consumption of 2 liters per minute. If oxygen was consumed faster (high workload), a regular scrubber was needed.
Cryogenic rebreathers were widely used in Soviet oceanography
in the period 1980 to 1990.
exhaled by the diver. Attached to the loop there will be at least one valve allowing injection of gases, such as oxygen and perhaps a diluting gas, from a gas source into the loop. There may be valves allowing venting of gas from the loop.
Most modern rebreathers have a twin hose mouthpiece or breathing mask where the direction of flow of gas through the loop is controlled by one-way valves. Some have a single pendulum hose, where the inhaled and exhaled gas passes through the same tube in opposite directions. The mouthpiece often has a valve letting the diver take the mouthpiece from the mouth while underwater or floating on the surface without water getting into the loop. Many rebreathers have "water traps" in the counterlungs, to stop large volumes of water from entering the loop if the diver removes the mouthpiece underwater without closing the valve, or if the diver's lips get slack letting water leak in. Regardless of whether the rebreather in question has the facility to trap any ingress of water, any training on a rebreather will feature procedures for removing any excess water.
. Depending on the rebreather design variant, the oxygen source will either be pure or a breathing gas
mixture.
Pure oxygen is not considered to be safe for recreational diving deeper than 6 meters, so recreational rebreathers and many professional diving rebreathers also have a cylinder of diluent
gas. This diluent cylinder may be filled with compressed air or another diving gas mix such as nitrox or trimix. The diluent reduces the percentage of oxygen breathed and increases the maximum operating depth
of the rebreather. It is important that the diluent is not an oxygen-free gas, such as pure nitrogen or helium, and is breathable; it may be used in an emergency either to flush the loop with breathable gas or as a bailout.
, which removes the carbon dioxide from the gas mixture and leaves the oxygen and other gases available for re-breathing.
Some absorbent chemical designed for diving applications are Sofnolime, Drager
sorb, or Sodasorb. Some systems use a prepackaged Reactive Plastic Curtain (RPC) based cartridge: Reactive Plastic Curtain (RPC) was first used between Micropore Inc. and the US Navy to describe Micropore's absorbent curtains for emergency submarine use, and then more recently RPC has been used on the web to describe their Reactive Plastic Cartridges – ExtendAir.
The carbon dioxide
passing through the scrubber absorbent is removed when it reacts with the absorbent in the canister; this chemical reaction
is exothermic
. This reaction occurs along a "front" which is a cross section of the canister, of the unreacted soda lime that is exposed to carbon dioxide-laden gas. This front moves through the scrubber canister, from the gas input end to the gas output end, as the reaction consumes the active ingredients. However, this front would be a wide zone, because the carbon dioxide in the gas going through the canister needs time to reach the surface of a grain of absorbent, and then time to penetrate to the middle of each grain of absorbent as the outside of the grain becomes exhausted.
In larger environments, such as recompression chambers, a fan is used to pass gas through the canister.
There are several ways that the scrubber may fail or become less efficient:
of oxygen (ppO2) in the mix from getting too low (causing hypoxia
) or too high (causing oxygen toxicity
). If not enough new oxygen is being added, the proportion of oxygen in the loop may be too low to support life. In humans, the urge to breathe is normally caused by a build-up of carbon dioxide in the blood, rather than lack of oxygen. The resulting serious hypoxia causes sudden blackout with little or no warning. This makes hypoxia
a deadly problem for rebreather divers.
In many rebreathers the diver can control the gas mix and volume in the loop manually by injecting each of the different available gases to the loop and by venting the loop. The loop often has a pressure relief valve to prevent over-pressure injuries caused by over-pressure of the loop.
 In some early rebreathers the diver had to manually open and close the valve to the oxygen cylinder to refill the counter-lung each time. In others the oxygen flow is kept constant by a pressure-reducing flow valve like the valves on blowtorch cylinders; the set also has a manual on/off valve called a bypass. In some modern rebreathers, the pressure in the breathing bag controls the oxygen flow like the demand valve in open-circuit scuba; for example, trying to breathe in from an empty bag makes the cylinder release more gas. Most modern closed-circuit rebreathers have electro-galvanic fuel cell
In some early rebreathers the diver had to manually open and close the valve to the oxygen cylinder to refill the counter-lung each time. In others the oxygen flow is kept constant by a pressure-reducing flow valve like the valves on blowtorch cylinders; the set also has a manual on/off valve called a bypass. In some modern rebreathers, the pressure in the breathing bag controls the oxygen flow like the demand valve in open-circuit scuba; for example, trying to breathe in from an empty bag makes the cylinder release more gas. Most modern closed-circuit rebreathers have electro-galvanic fuel cell
sensors and onboard electronics, which monitor the ppO2, injecting more oxygen if necessary or issuing an audible warning to the diver if the ppO2 reaches dangerously high or low levels.
Underwater, the position of the breathing bag, on the chest, over the shoulders, or on the back, has an effect on the ease of breathing. This is due to the pressure difference between the counterlung and the diver's lung caused by the vertical distance between the two. It is easier to inhale from a front mounted counterlung and exhale to a back mounted counterlung for diver swimming facedown and horizontally.
The design of the rebreathers' counterlungs can also affect the swimming diver's streamlining
due to location of the counterlungs themselves. Some are designed as over-the-shoulder lungs (e.g. Innerspace Systems Megalodon), while others incorporate the counter lungs into a solid case (e.g. The KISS Classic).
For use out of water, this does not matter so much: for example, in an industrial version of the Siebe Gorman Salvus
the breathing bag hangs down by the left hip.
A rebreather whose counterlung is rubber
and not in an enclosed casing, should be sheltered from sunlight
when not in use, to prevent the rubber from perishing due to UV light.
Although some rebreather divers—referred to as "alpinists"—do not carry bailouts, bailout strategy becomes a crucial part of dive planning, particularly for long dives and deeper dives in technical diving
. Often the planned dive is limited by the capacity of the bailout and not the capacity of the rebreather.
Several types of bailout are possible:
, which is used for mine rescue
, to keep grit and stones out of its working, is completely sealed, except for a large vent panel covered with metal mesh
, and holes for the oxygen cylinder's on/off valve and the cylinder pressure gauge. Underwater the casing also serves for streamlining
, e.g. in the IDA71 and Cis-Lunar
.
from 1998 through 2004. Investigations into rebreather deaths focus on three main areas: medical, equipment, and procedural.
In mountaineering, closed-circuit rebreathers are ideal to treat various altitude related illnesses as the user is brought back to sea level in terms of oxygen partial pressure (pp). The danger is that a sick climber using a rebreather might become unconscious. Because an absolute atmospheric seal is required for rebreathers to work correctly, such a seal could conceivably cause an unconscious user to suffocate when the oxygen ran out or the scrubber became exhausted. (Because there has been very little use of mountaineering rebreathers, this danger is still only theoretical.)
of oxygen in the diluent alone would not cause hypoxia
or hyperoxia
, such as when using a normoxic diluent and observing the diluent's maximum operating depth
. The technique involves simultaneously venting the loop and injecting diluent. This flushes out the old mix and replaces it with a known proportion of oxygen
s, rebreathers have some disadvantages including expense, complexity of operation and maintenance, and fewer failsafes. A malfunctioning rebreather can supply a gas mixture which contains too little oxygen to sustain life, or it may allow carbon dioxide
to build up to dangerous levels. Typically rebreathers try to solve these problems by monitoring the system with electronics, sensors and alarm systems. These are expensive and susceptible to failure, improper configuration and misuse.
The bailout requirement of rebreather diving can sometimes also require a rebreather diver to carry almost as much bulk of cylinders
as an open-circuit diver so the diver can complete the necessary decompression stops if the rebreather fails completely. Some rebreather divers prefer not to carry enough bailout for a safe ascent breathing open circuit, but instead rely on the rebreather, believing that an irrecoverable rebreather failure is very unlikely. This practice is known as alpinism or alpinist diving and is generally maligned due to the perceived extremely high risk of death if the rebreather fails.
Breathing set
*Scuba set, used underwater*Rebreather, reprocesses exhaled air*Surface supplied diving, fed from the surface*Self-contained breathing apparatus, used out of water, worn by rescue workers, firefighters, and others*Spacesuit, used in space...
that provides a breathing gas
Breathing gas
Breathing gas is a mixture of gaseous chemical elements and compounds used for respiration.Air is the most common and only natural breathing gas...
containing oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and recycled exhaled gas. This recycling reduces the volume of breathing gas used, making a rebreather lighter and more compact than an open-circuit breathing set
Breathing set
*Scuba set, used underwater*Rebreather, reprocesses exhaled air*Surface supplied diving, fed from the surface*Self-contained breathing apparatus, used out of water, worn by rescue workers, firefighters, and others*Spacesuit, used in space...
for the same duration in environments where humans cannot safely breathe from the atmosphere. In the armed forces
Armed forces
The armed forces of a country are its government-sponsored defense, fighting forces, and organizations. They exist to further the foreign and domestic policies of their governing body, and to defend that body and the nation it represents from external aggressors. In some countries paramilitary...
it is sometimes called "CCUBA" (Closed Circuit Underwater Breathing Apparatus).
Rebreather technology is used in many environments:
- Underwater – where it is sometimes known as CCR = "closed circuit rebreather", "closed circuit scubaScuba setA scuba set is an independent breathing set that provides a scuba diver with the breathing gas necessary to breathe underwater during scuba diving. It is much used for sport diving and some sorts of work diving....
", "semi closed scuba", SCR = "semi closed rebreather", or CCUBA = "closed circuit underwater breathing apparatus", as opposed to Aqua-LungAqua-lungAqua-Lung was the original name of the first open-circuit free-swimming underwater breathing set in reaching worldwide popularity and commercial success...
-type equipment, which is known as "open circuit scuba". - Mine rescueMine rescueMine rescue is the very specialized job of rescuing miners and others who have become trapped or injured underground in mines because of mining accidents and disasters such as explosions caused by firedamp, roof falls or floods.- Expert volunteers :...
and in industry – where poisonous gases may be present or oxygen may be absent. - Crewed spacecraftSpacecraftA spacecraft or spaceship is a craft or machine designed for spaceflight. Spacecraft are used for a variety of purposes, including communications, earth observation, meteorology, navigation, planetary exploration and transportation of humans and cargo....
and space suitSpace suitA space suit is a garment worn to keep an astronaut alive in the harsh environment of outer space. Space suits are often worn inside spacecraft as a safety precaution in case of loss of cabin pressure, and are necessary for extra-vehicular activity , work done outside spacecraft...
s – outer spaceOuter spaceOuter space is the void that exists between celestial bodies, including the Earth. It is not completely empty, but consists of a hard vacuum containing a low density of particles: predominantly a plasma of hydrogen and helium, as well as electromagnetic radiation, magnetic fields, and neutrinos....
is, effectively, a vacuumVacuumIn everyday usage, vacuum is a volume of space that is essentially empty of matter, such that its gaseous pressure is much less than atmospheric pressure. The word comes from the Latin term for "empty". A perfect vacuum would be one with no particles in it at all, which is impossible to achieve in...
with no oxygen to support life. - Hospital anaesthesia breathing systems – to supply controlled proportions of gases to patients without letting anaesthetic gas get into the atmosphere that the staff breathe.
- HimalayanHimalayasThe Himalaya Range or Himalaya Mountains Sanskrit: Devanagari: हिमालय, literally "abode of snow"), usually called the Himalayas or Himalaya for short, is a mountain range in Asia, separating the Indian subcontinent from the Tibetan Plateau...
mountaineering. Both chemical and compressed oxygen has been used in experimental closed-circuit oxygen systems—the first on Mt. Everest in 1938. A high rate of system failures due to extreme cold has not been solved. - SubmarineSubmarineA submarine is a watercraft capable of independent operation below the surface of the water. It differs from a submersible, which has more limited underwater capability...
s and hyperbaric oxygen therapyHyperbaric oxygen therapyHyperbaric medicine, also known as hyperbaric oxygen therapy , is the medical use of oxygen at a level higher than atmospheric pressure. The equipment required consists of a pressure chamber, which may be of rigid or flexible construction, and a means of delivering 100% oxygen...
chambers – where the gas in the habitat must remain safe. Here the rebreather is big and is connected to the air in the habitat.
Theory
As a person breathes, the body consumes oxygenOxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and makes carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
. At shallow depths, a person with an open-circuit breathing set typically only uses about a quarter of the oxygen in the air that is breathed in (4%–5% of the inspired volume). The remaining oxygen is exhaled along with nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
and carbon dioxide. As the diver goes deeper, roughly the same quantity of oxygen is used, which represents an increasingly smaller fraction of the compressed air breathed in. Because exhaled air can contain as much as 79% nitrogen (which is not utilized in the body) and 16% (or more) unused oxygen, every exhaled breath from an open-circuit scuba set represents at least 95% wasted, potentially useful gas volume, which has to be replaced from the air supply.
The rebreather recirculates the exhaled gas for re-use and does not discharge it to the atmosphere or water. It absorbs the carbon dioxide, which otherwise would accumulate and cause carbon dioxide poisoning. It removes the carbon dioxide by a process called scrubbing
Carbon dioxide scrubber
A carbon dioxide scrubber is a device which absorbs carbon dioxide . It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers...
. The rebreather adds oxygen, to replace the oxygen that was consumed. Thus, the gas in the rebreather's circuit remains breathable and supports life and the diver needs only a fraction of the gas that would be required for an open-circuit system.
History of rebreathers

- Around 1620: In EnglandEnglandEngland is a country that is part of the United Kingdom. It shares land borders with Scotland to the north and Wales to the west; the Irish Sea is to the north west, the Celtic Sea to the south west, with the North Sea to the east and the English Channel to the south separating it from continental...
, Cornelius DrebbelCornelius DrebbelCornelis Jacobszoon Drebbel was the Dutch builder of the first navigable submarine in 1620. Drebbel was an innovator who contributed to the development of measurement and control systems, optics and chemistry....
made an early oar-powered submarineSubmarineA submarine is a watercraft capable of independent operation below the surface of the water. It differs from a submersible, which has more limited underwater capability...
. To re-oxygenate the air inside it, he likely generated oxygen by heating saltpetre (potassium nitratePotassium nitratePotassium nitrate is a chemical compound with the formula KNO3. It is an ionic salt of potassium ions K+ and nitrate ions NO3−.It occurs as a mineral niter and is a natural solid source of nitrogen. Its common names include saltpetre , from medieval Latin sal petræ: "stone salt" or possibly "Salt...
) in a metal pan to emit oxygen. Heating turns the saltpetre into potassium oxideOxideAn oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
or hydroxideHydroxideHydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
, which absorbs carbon dioxide from the air. That may explain why Drebbel's men were not affected by carbon dioxide build-upHypercapniaHypercapnia or hypercapnea , also known as hypercarbia, is a condition where there is too much carbon dioxide in the blood...
as much as would be expected. If so, he accidentally made a crude rebreather more than two centuries before Saint Simon Sicard's patent.
- 1808: The oldest known rebreather based on carbon dioxide absorption was patented in FranceFranceThe French Republic , The French Republic , The French Republic , (commonly known as France , is a unitary semi-presidential republic in Western Europe with several overseas territories and islands located on other continents and in the Indian, Pacific, and Atlantic oceans. Metropolitan France...
by Sieur (old French for "sir" or "Mister") Touboulic from BrestBrest, FranceBrest is a city in the Finistère department in Brittany in northwestern France. Located in a sheltered position not far from the western tip of the Breton peninsula, and the western extremity of metropolitan France, Brest is an important harbour and the second French military port after Toulon...
, mechanicMechanicA mechanic is a craftsman or technician who uses tools to build or repair machinery.Many mechanics are specialized in a particular field such as auto mechanics, bicycle mechanics, motorcycle mechanics, boiler mechanics, general mechanics, industrial maintenance mechanics , air conditioning and...
in the Napoleon's Imperial Navy. This early rebreather design worked with an oxygen reservoir, the oxygen being delivered progressively by the diver himself and circulating in a closed circuit through a sponge soaked in lime waterLime waterLimewater is the common name for saturated calcium hydroxide solution. It is sparsely soluble. Its chemical formula is Ca2. Since calcium hydroxide is only sparsely soluble, i.e. ca. 1.5 g per liter at 25 °C, there is no visible distinction to clear water. Attentive observers will notice a slightly...
. Touboulic called his invention Ichtioandre (Greek for 'fish-man'). There's no evidence of a prototype having been manufactured.
- 1849: Patent for the oldest known prototype of a rebreather also used an oxygen reservoir, granted to the Frenchman Pierre Aimable De Saint Simon Sicard.
- 1853: Professor T. Schwann designed a rebreather in BelgiumBelgiumBelgium , officially the Kingdom of Belgium, is a federal state in Western Europe. It is a founding member of the European Union and hosts the EU's headquarters, and those of several other major international organisations such as NATO.Belgium is also a member of, or affiliated to, many...
; he exhibited it in Paris in 1878. It had a big backpack oxygen tank at pressure about 13.333 bars, and two scrubbers containing sponges soaked in caustic soda.
- 1878: Henry FleussHenry FleussHenry Albert Fleuss was a pioneering diving engineer, and Master Diver for Siebe, Gorman & Co. of London.Fleuss was born in Marlborough, Wiltshire in 1851....
invented a rebreather using stored oxygen and absorption of carbon dioxide by an absorbent (here rope yarn soaked in caustic potash solution), to rescue mineMiningMining is the extraction of valuable minerals or other geological materials from the earth, from an ore body, vein or seam. The term also includes the removal of soil. Materials recovered by mining include base metals, precious metals, iron, uranium, coal, diamonds, limestone, oil shale, rock...
workers who were trapped by water.
- About 1900: The Davis Escape SetDavis Submerged Escape ApparatusThe Davis Submerged Escape Apparatus , was an early type of oxygen rebreather invented in 1910 by Sir Robert Davis, head of Siebe Gorman and Co. Ltd., inspired by the earlier Fleuss system...
was designed in Britain for escape from sunken submarines. It was the first rebreather which was practical for use and produced in quantity. Various industrial oxygen rebreathers (e.g. the Siebe Gorman SalvusSiebe Gorman SalvusThe Siebe Gorman Salvus is a light oxygen rebreather for industrial use or in shallow diving. Its duration on a filling is 30 to 40 minutes. It was very common in Britain during World War II and for a long time afterwards...
and the Siebe Gorman ProtoSiebe Gorman ProtoThe Proto is a type of rebreather that was made by Siebe Gorman. It was an industrial breathing set and not suitable for diving. It was made from 1914 or earlier to the 1960s or later. .Its breathing bag was worn on the chest...
, both invented in the early 1900s) were derived from it.
- 1903 to 1907: Professor Georges Jaubert invented Oxylithe, which is a form of sodium peroxideSodium peroxideSodium peroxide is the inorganic compound with the formula Na2O2. This solid is the product when sodium is burned with oxygen. It is a strong base and a potent oxidizing agent. It exists in several hydrates and peroxyhydrates including Na2O2·2H2O2·4H2O, Na2O2·2H2O, Na2O2·2H2O2, and...
(Na2O2) or sodium dioxide (NaO2). As it absorbs carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(e.g. in a rebreather's scubber) it emits oxygen. - 1907: Oxylithe was used in the first filming of Twenty Thousand Leagues Under the SeaTwenty Thousand Leagues Under the SeaTwenty Thousand Leagues Under the Sea is a classic science fiction novel by French writer Jules Verne published in 1870. It tells the story of Captain Nemo and his submarine Nautilus as seen from the perspective of Professor Pierre Aronnax...
. - 1907: This link shows a Draeger rebreather used for mines rescue.
- In 1909 Captain S.S. Hall, R.N., and Dr. O. Rees, R.N., developed a submarine escape apparatus using Oxylithe; the Royal Navy accepted it. It was used for shallow water diving but never in a submarine escape;
- 1912: The first recorded mass production of rebreathers started with the DrägerDrägerThe Drägerwerk AG is a German company based in Lübeck which makes breathing and protection equipment, gas detection and analysis systems, and noninvasive patient monitoring technologies. Customers include hospitals, fire departments and diving companies....
rebreathers, invented some years earlier by an engineer of the Dräger company, Hermann Stelzner. The Dräger rebreathers, especially the DM20 and DM40 model series, were those used by the German helmet diversStandard diving dressA standard diving dress consists of a metallic diving helmet, an airline or hose from a surface supplied diving air pump, a canvas diving suit, diving knife and boots...
and German frogmenFrogmanA frogman is someone who is trained to scuba diving or swim underwater in a military capacity which can include combat. Such personnel are also known by the more formal names of combat diver or combatant diver or combat swimmer....
during World War IIWorld War IIWorld War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
.
- 1930's: ItalianItalyItaly , officially the Italian Republic languages]] under the European Charter for Regional or Minority Languages. In each of these, Italy's official name is as follows:;;;;;;;;), is a unitary parliamentary republic in South-Central Europe. To the north it borders France, Switzerland, Austria and...
sport spearfishers used rebreathers systematically. This practice came to the attention of the Italian NavyItalian NavyItalian Navy may refer to:* Pre-unitarian navies of the Italian states* Regia Marina, the Royal Navy of the Kingdom of Italy * Italian Navy , the navy of the Italian Republic...
, which developed its frogman unit Decima Flottiglia MASDecima Flottiglia MASThe Decima Flottiglia MAS was an Italian commando frogman unit of the Regia Marina created during the Fascist regime.The acronym MAS also refers to various light torpedo boats used by the Regia Marina during World...
, which was used effectively in World War IIWorld War IIWorld War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
.
- World War IIWorld War IIWorld War II, or the Second World War , was a global conflict lasting from 1939 to 1945, involving most of the world's nations—including all of the great powers—eventually forming two opposing military alliances: the Allies and the Axis...
: Captured Italian frogmen's rebreathers influenced design of British rebreathers. Many British frogmen's breathing sets' oxygen cylinders were German pilot's oxygen cylinders recovered from shot-down German LuftwaffeLuftwaffeLuftwaffe is a generic German term for an air force. It is also the official name for two of the four historic German air forces, the Wehrmacht air arm founded in 1935 and disbanded in 1946; and the current Bundeswehr air arm founded in 1956....
planes. Those first breathing sets may have been modified Davis Submarine Escape SetDavis Submerged Escape ApparatusThe Davis Submerged Escape Apparatus , was an early type of oxygen rebreather invented in 1910 by Sir Robert Davis, head of Siebe Gorman and Co. Ltd., inspired by the earlier Fleuss system...
s; their fullface masks were the type intended for the Siebe Gorman SalvusSiebe Gorman SalvusThe Siebe Gorman Salvus is a light oxygen rebreather for industrial use or in shallow diving. Its duration on a filling is 30 to 40 minutes. It was very common in Britain during World War II and for a long time afterwards...
. But in later operations different designs were used, leading to a fullface mask with one big face window, at first oval like in this image, and later rectangular (mostly flat, but the ends curved back to allow more vision sideways). Early British frogman's rebreathers had rectangular breathing bags on the chest like Italian frogman's rebreathers; later British frogman's rebreathers had a square recess in the top so they could extend further up onto his shoulders; in front they had a rubber collar that was clamped around the absorbent canister, as in the illustration below.- Some British armed forces divers used bulky thick diving suits called Sladen suitSladen SuitThe Sladen Suit was a heavy type of British divers' drysuit made by Siebe Gorman. It is entered by a wide rubber tube at the umbilicus: this tube is folded and tied off before the diver dives. It was used by British manned torpedo riders and for general underwater work.It was sometimes nicknamed...
s; one version of it had a flip-up single window for both eyes to let the user get binocularsBinocularsBinoculars, field glasses or binocular telescopes are a pair of identical or mirror-symmetrical telescopes mounted side-by-side and aligned to point accurately in the same direction, allowing the viewer to use both eyes when viewing distant objects...
to his eyes when on the surface.
- Some British armed forces divers used bulky thick diving suits called Sladen suit
- Early 1940s: US Navy rebreathers were developed by Dr. Christian J. LambertsenChristian J. LambertsenChristian James Lambertsen was an American environmental medicine and diving medicine specialist who was principally responsible for developing the United States Navy frogmen's rebreathers in the early 1940s for underwater warfare...
for underwater warfare and is considered by the US Navy as "the father of the frogmen". Lambertsen held the first closed-circuit oxygen rebreather course in the United States for the Office of Strategic ServicesOffice of Strategic ServicesThe Office of Strategic Services was a United States intelligence agency formed during World War II. It was the wartime intelligence agency, and it was a predecessor of the Central Intelligence Agency...
maritime unit at the Naval AcademyUnited States Naval AcademyThe United States Naval Academy is a four-year coeducational federal service academy located in Annapolis, Maryland, United States...
on 17 May 1943.
- c.1960 to c.1990: In this period in Britain there was very little rebreather use by civilians, and no easy way for the general public to obtain rebreathers, and the BSAC forbad rebreather use by its members. The Italian firms PirelliPirelliPirelli & C. SpA is a diverse multinational company based in Milan, Italy. The company, the world’s fifth largest tyre manufacturer, is present in over 160 countries, has 20 manufacturing sites around the world and a network of around 10,000 distributors and retailers.Founded in Milan in 1872,...
and Cressi-SubCressi-SubCressi-Sub is an Italian manufacturer of scuba gear and one of the oldest underwater diving companies presently existing. Officially, the company was founded in 1946 by brothers Egidio and Nanni Cressi, although they had already begun small-scale manual production in 1943.The history of underwater...
at first each sold a model of sport diving rebreather, but after a while discontinued those models. This image is of a rebreather home-made from a FenzyFenzyFenzy is a scuba diving and industrial breathing equipment making firm. It started in or before 1920.In 1961 its member Maurice Fenzy invented the first inflatable divers' lifejacket : see Buoyancy compensator #History.They made rebreathers and inflatable divers' lifejackets.-External links:Fenzy...
diver's lifejacket (probably in New ZealandNew ZealandNew Zealand is an island country in the south-western Pacific Ocean comprising two main landmasses and numerous smaller islands. The country is situated some east of Australia across the Tasman Sea, and roughly south of the Pacific island nations of New Caledonia, Fiji, and Tonga...
) in 1980. Some better-quality rebreathers were home-made by cave divers to penetrate sumpsSump (cave)Sump is a term used in caving to describe a submerged passage in a cave. A sump may be static, with no inward or outward flow, or active, with continuous through-flow...
.
- 1989: The Communist Bloc falls and the Cold WarCold WarThe Cold War was the continuing state from roughly 1946 to 1991 of political conflict, military tension, proxy wars, and economic competition between the Communist World—primarily the Soviet Union and its satellite states and allies—and the powers of the Western world, primarily the United States...
ends (see Fall of Communism and dissolution of the Soviet UnionDissolution of the Soviet UnionThe dissolution of the Soviet Union was the disintegration of the federal political structures and central government of the Union of Soviet Socialist Republics , resulting in the independence of all fifteen republics of the Soviet Union between March 11, 1990 and December 25, 1991...
), and with it the risk of future attack by Communist Bloc forces including by their combat diversFrogmanA frogman is someone who is trained to scuba diving or swim underwater in a military capacity which can include combat. Such personnel are also known by the more formal names of combat diver or combatant diver or combat swimmer....
. After that, the world's armed forces had less reason to requisition rebreather patentPatentA patent is a form of intellectual property. It consists of a set of exclusive rights granted by a sovereign state to an inventor or their assignee for a limited period of time in exchange for the public disclosure of an invention....
s submitted by civilians, and sport diving automatic and semi-automatic mixture rebreathers start to appear.
Efficiency advantages
The main advantage of the rebreather over other breathing equipment is the rebreather's economical use of gas. With open circuit scuba, the entire breath is expelled into the surrounding water when the diver exhales. A breath inhaled from an open circuit scuba system whose cylinders are filled with ordinary air is about 21% oxygen. When that breath is exhaled back into the surrounding environment, it has an oxygen level in the range of 15 to 16% when the diver is at atmospheric pressure. This leaves the available oxygen utilization at about 25%; the remaining 75% is lost. As the remaining 79% of the breathing gas (mostly nitrogenNitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
) is inert, the diver on open-circuit scuba only uses about 5% of his cylinders' contents.
At depth, the advantage of a rebreather is even more marked. Since the generation of CO2 is directly related to the body's consumption of O2 (about ~99.5% of O2 is converted to CO2 on exhalation), the amount of O2 consumption doesn't change, therefore CO2 generation doesn't change. This means that at depth, the diver is not using any more of the O2 gas supply than when shallower. This is a marked difference from open circuit where the amount of gas consumed increases as depth increases.
Feasibility advantages
Long or deep dives using open circuit equipment may not be feasible as there are limits to the number and weight of diving cylinderDiving cylinder
A diving cylinder, scuba tank or diving tank is a gas cylinder used to store and transport high pressure breathing gas as a component of a scuba set. It provides gas to the scuba diver through the demand valve of a diving regulator....
s the diver can carry. The economy of gas consumption is also useful when the gas mix being breathed contains expensive gases, such as helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
. In normal use, only oxygen is consumed: small volumes of expensive inert gases are reused during (only) one dive, due to venting of the gas on ascent. For example, a closed circuit rebreather diver effectively doesn't use any of their diluent gas once they've reached the bottom phase of the dive; they could turn off their diluent. On ascent, no diluent is added, however most of that in circuit is lost. A very small amount of trimix would then last for many dives. It is not uncommon for a 3 litre (19 cubic foot) diluent cylinder to last for eight 40 m (131.2 ft) dives.
Other advantages
Except on ascent, closed circuit rebreathers produce no bubbles and make no bubble noise and much less gas hissing, unlike open-circuit scuba; this can conceal military divers and allow divers engaged in marine biologyMarine biology
Marine biology is the scientific study of organisms in the ocean or other marine or brackish bodies of water. Given that in biology many phyla, families and genera have some species that live in the sea and others that live on land, marine biology classifies species based on the environment rather...
and underwater photography
Underwater photography
Underwater photography is the process of taking photographs while under water. It is usually done while scuba diving, but can be done while snorkeling or swimming.-Overview:...
to avoid alarming marine animals and thereby get closer to them. This lack of exhale also allows shipwreck divers to enter enclosed areas on sunken ships and avoid slowly filling them with air, which then supports the growth of rust.
The fully closed circuit rebreather is able to minimise the proportion of inert gases in the breathing mix, and therefore minimise the decompression requirements of the diver, by maintaining a specific and relatively high oxygen partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
(ppO2) at all depths. The breathing gas in a rebreather is warmer and more moist than the dry and cold gas from open circuit equipment making it more comfortable to breathe on long dives and causing less dehydration in the diver.
Most modern rebreathers have a system of very sensitive oxygen sensors, which allow the diver to adjust the partial pressure of oxygen. This can offer a dramatic advantage at the end of deeper dives, where a diver can raise the partial pressure of oxygen somewhat at shallower depth, in order to shorten decompression times. Care must be taken that the ppO2 is not set to a level where it can become toxic though. Research has shown that a ppO2 of 1.6 bar is toxic with extended exposure
One major difference between rebreather diving and open-circuit scuba diving is in keeping neutral buoyancy. When an open-circuit scuba diver inhales, a quantity of highly compressed gas from his cylinder is reduced in pressure by a regulator, and enters the lungs at a much higher volume than it occupied in the cylinder. This means that the diver has a tendency to rise slightly with each inhalation, and lower slightly with each exhalation. This does not happen to a rebreather diver, because the diver is circulating a roughly constant volume of gas between his lungs and the breathing bag.
Oxygen rebreather

Navy
A navy is the branch of a nation's armed forces principally designated for naval and amphibious warfare; namely, lake- or ocean-borne combat operations and related functions...
from the early twentieth century. Oxygen rebreathers can be remarkably simple designs, and their invention predates that of open-circuit scuba. The only gas that it supplies is oxygen. As pure oxygen is toxic
Oxygen toxicity
Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen at elevated partial pressures. It is also known as oxygen toxicity syndrome, oxygen intoxication, and oxygen poisoning...
when inhaled at pressure, oxygen rebreathers are currently limited to a depth of 6 meters (20 ft); some say 9 meters (30 ft). In the past they have been used deeper (up to 20 meters) but such dives were more risky than what is now considered acceptable. Oxygen rebreathers are also sometimes used when decompressing from a deep open-circuit dive, as breathing pure oxygen makes the nitrogen diffuse out of the blood more rapidly.
The diving pioneer Hans Hass
Hans Hass
Hans Hass is a diving pioneer known mainly for his documentaries about sharks, the energon theory, and his commitment, later in life, to the protection of the environment. He was born in Vienna, Austria.-Early years:...
used Dräger
Dräger
The Drägerwerk AG is a German company based in Lübeck which makes breathing and protection equipment, gas detection and analysis systems, and noninvasive patient monitoring technologies. Customers include hospitals, fire departments and diving companies....
oxygen rebreathers in the early 1940s.
In some rebreathers, e.g. the Siebe Gorman Salvus
Siebe Gorman Salvus
The Siebe Gorman Salvus is a light oxygen rebreather for industrial use or in shallow diving. Its duration on a filling is 30 to 40 minutes. It was very common in Britain during World War II and for a long time afterwards...
, the oxygen cylinder has two first stages in parallel. One is constant flow; the other is a plain on-off valve called a bypass
Bypass (valve)
In rebreather breathing sets, a bypass is a hand-operated valve that can be used to let more oxygen into the breathing system, by-passing the cylinder's flow rate control valve....
; both feed into the same exit pipe which feeds the breathing bag. In the Salvus there is no second stage and the gas is turned on and off at the cylinder. Some simple oxygen rebreathers had no constant-flow valve, but only the bypass, and the diver had to operate the valve at intervals to refill the breathing bag as he used the oxygen.
Oxygen rebreathers are no longer commonly used in diving because of the depth limit imposed by oxygen toxicity. However, they are still the most commonly used for industrial applications on the surface, (SCBA) such as in mines, due to their simplicity and compact size.
Semi-closed circuit rebreather

Maximum operating depth
In technical diving and nitrox diving, the maximum operating depth of a breathing gas is the depth at which the partial pressure of oxygen of the gas mix exceeds a safe limit...
than oxygen rebreathers and are fairly simple and cheap.
Semi-closed circuit equipment generally supplies one breathing gas such as air or nitrox or trimix. The gas is injected into the loop at a constant rate to replenish oxygen consumed from the loop by the diver. Excess gas must be constantly vented from the loop in small volumes to make space for fresh, oxygen-rich gas. As the oxygen in the vented gas cannot be separated from the inert gas, semi-closed circuit is wasteful of oxygen.
The diver must fill the cylinders with gas mix that has a maximum operating depth that is safe for the depth of the dive being planned.
As the amount of oxygen required by the diver increases with work rate, the gas injection rate must be carefully chosen and controlled to prevent unconsciousness
Unconsciousness
Unconsciousness is the condition of being not conscious—in a mental state that involves complete or near-complete lack of responsiveness to people and other environmental stimuli. Being in a comatose state or coma is a type of unconsciousness. Fainting due to a drop in blood pressure and a...
in the diver due to hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
. A higher gas injection rate reduces the likelihood of hypoxia but consumes more gas and wastes more oxygen.
Fully closed circuit rebreather

The major task of the fully closed circuit rebreather is to control the oxygen concentration, known as the oxygen partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
, in the loop and to warn the diver if it is becoming dangerously low or high. The concentration of oxygen in the loop depends on two factors: depth and the proportion of oxygen in the mix. Too low a concentration of oxygen results in hypoxia leading to unconsciousness and ultimately death
Death
Death is the permanent termination of the biological functions that sustain a living organism. Phenomena which commonly bring about death include old age, predation, malnutrition, disease, and accidents or trauma resulting in terminal injury....
. Too high a concentration of oxygen results in hyperoxia, leading to oxygen toxicity
Oxygen toxicity
Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen at elevated partial pressures. It is also known as oxygen toxicity syndrome, oxygen intoxication, and oxygen poisoning...
, a condition causing convulsions which can make the diver lose the mouthpiece when they occur underwater, and can lead to drowning
Drowning
Drowning is death from asphyxia due to suffocation caused by water entering the lungs and preventing the absorption of oxygen leading to cerebral hypoxia....
.
In fully automatic closed-circuit systems, a mechanism injects oxygen into the loop when it detects that the partial pressure of oxygen in the loop has fallen below the required level. Often this mechanism is electrical and relies on oxygen sensitive electro-galvanic fuel cell
Electro-galvanic fuel cell
An electro-galvanic fuel cell is an electrical device, one form of which is commonly used to measure the concentration of oxygen gas in scuba diving and medical equipment....
s called “ppO2 meters” to measure the concentration of oxygen in the loop.
The diver may be able to manually control the mixture by adding diluent gas or oxygen. Adding diluent can prevent the loop's gas mixture becoming too oxygen rich. Manually adding oxygen is risky as additional small volumes of oxygen in the loop can easily raise the partial pressure of oxygen to dangerous levels.
Rebreathers using an absorbent that releases oxygen
There have been a few rebreather designs (e.g. the Oxylite) which had an absorbent canister filled with potassium superoxide, which gives off oxygen as it absorbs carbon dioxide: 4KO2 + 2CO2 = 2K2CO3 + 3O2; it had a very small oxygen cylinder to fill the loop at the start of the dive. This system is dangerous because of the explosively hot reaction that happens if water gets on the potassium superoxide. The Russian IDA71 military and naval rebreatherRussian IDA71 military and naval rebreather
The Russian IDA71 military and naval rebreather is an oxygen rebreather intended for use by naval and military divers. As supplied it is in a plain backpack harness with no buoyancy aid. Its casing is metal, not plastic. It has a small optional diluent cylinder which can be clipped on its outside...
was designed to be run in this mode or as an ordinary rebreather.
Tests on the IDA71 at the United States Navy Experimental Diving Unit
United States Navy Experimental Diving Unit
The United States Navy Experimental Diving Unit is the primary source of diving and hyperbaric operational guidance for the US Navy...
in Panama City, Florida
Panama City, Florida
-Personal income:The median income for a household in the city was $31,572, and the median income for a family was $40,890. Males had a median income of $30,401 versus $21,431 for females. The per capita income for the city was $17,830...
showed that the IDA71 could give significantly longer dive time with superoxide in one of the canisters than without.
Rebreathers which store liquid oxygen

- Aerophor.
- Aerorlox http://www.healeyhero.co.uk/rescue/glossary/aerorlox.htm
- Cryogenic rebreather: see below.
Cryogenic rebreather
A cryogenic rebreather has a tank of liquid oxygen and no absorbent canister. The carbon dioxide is frozen out in a "snow box" by the cold produced as the liquid oxygen expands to gas as the oxygen is used and is replaced from the oxygen tank.A cryogenic rebreather called the S-1000 was built around or soon after 1960 by Sub-Marine Systems Corporation. It had a duration of 6 hours and a maximum dive depth of 200 meters sea water. Its ppO2 could be set to anything from 0.2 bar to 2 bar without electronics, by controlling the temperature of the liquid oxygen, thus controlling the equilibrium pressure of oxygen gas above the liquid. The diluent could be either liquid nitrogen
Liquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
or helium depending on the depth of the dive. The set could freeze out 230 grams of carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
per hour from the loop, corresponding to an oxygen consumption of 2 liters per minute. If oxygen was consumed faster (high workload), a regular scrubber was needed.
Cryogenic rebreathers were widely used in Soviet oceanography
Oceanography
Oceanography , also called oceanology or marine science, is the branch of Earth science that studies the ocean...
in the period 1980 to 1990.
Other designs
- In the Siebe Gorman ProtoSiebe Gorman ProtoThe Proto is a type of rebreather that was made by Siebe Gorman. It was an industrial breathing set and not suitable for diving. It was made from 1914 or earlier to the 1960s or later. .Its breathing bag was worn on the chest...
the absorbent was in a flexible-walled compartment in the bottom of the breathing bag and not in a canister. - This link describes an experimental drysuit (with built-in hood and fullface mask) and rebreather combination where the drysuit acts as the breathing bag, like in an old Draeger standard diving suit variant which had a rebreather pack attached.
- Some British naval rebreathers (e.g. the Siebe Gorman CDBASiebe Gorman CDBAThe Clearance Divers Breathing Apparatus is a type of rebreather made by Siebe Gorman in England.The Royal Navy used it for many years. It was for underwater work rather than for combat diving. The main oxygen cylinders are on the diver's back. The oxygen cylinders at the front of the diver are...
) had a backpack weight pouch instead of the diver having a separate weight belt.
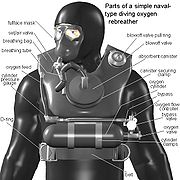
Parts of a rebreather

The loop
Although there are several design variations of diving rebreather, all types have a gas-tight loop that the diver inhales from and exhales into. The loop consists of components sealed together. The diver breathes through a mouthpiece or a fullface mask (or with industrial breathing sets, sometimes a mouth-and-nose mask). This is connected to one or more tubes bringing inhaled gas and exhaled gas between the diver and a counterlung or breathing bag. This holds gas when it is not in the diver's lungs. The loop also includes a scrubber containing carbon dioxide absorbent to remove from the loop the carbon dioxideCarbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
exhaled by the diver. Attached to the loop there will be at least one valve allowing injection of gases, such as oxygen and perhaps a diluting gas, from a gas source into the loop. There may be valves allowing venting of gas from the loop.
Most modern rebreathers have a twin hose mouthpiece or breathing mask where the direction of flow of gas through the loop is controlled by one-way valves. Some have a single pendulum hose, where the inhaled and exhaled gas passes through the same tube in opposite directions. The mouthpiece often has a valve letting the diver take the mouthpiece from the mouth while underwater or floating on the surface without water getting into the loop. Many rebreathers have "water traps" in the counterlungs, to stop large volumes of water from entering the loop if the diver removes the mouthpiece underwater without closing the valve, or if the diver's lips get slack letting water leak in. Regardless of whether the rebreather in question has the facility to trap any ingress of water, any training on a rebreather will feature procedures for removing any excess water.
Gas sources
A rebreather must have a source of oxygen to replenish that consumed by the diver. Nearly always, this oxygen is stored in a gas cylinderGas cylinder
A gas cylinder is a pressure vessel used to store gases at above atmospheric pressure. High pressure gas cylinders are also called bottles. Although they are sometimes colloquially called "tanks", this is technically incorrect, as a tank is a vessel used to store liquids at ambient pressure and...
. Depending on the rebreather design variant, the oxygen source will either be pure or a breathing gas
Breathing gas
Breathing gas is a mixture of gaseous chemical elements and compounds used for respiration.Air is the most common and only natural breathing gas...
mixture.
Pure oxygen is not considered to be safe for recreational diving deeper than 6 meters, so recreational rebreathers and many professional diving rebreathers also have a cylinder of diluent
Diluent
A diluent is a diluting agent.Certain fluids are too viscous to be pumped easily or too dense to flow from one particular point to the other. This can be problematic, because it might not be economically feasible to transport such fluids in this state.To ease this restricted movement, diluents...
gas. This diluent cylinder may be filled with compressed air or another diving gas mix such as nitrox or trimix. The diluent reduces the percentage of oxygen breathed and increases the maximum operating depth
Maximum operating depth
In technical diving and nitrox diving, the maximum operating depth of a breathing gas is the depth at which the partial pressure of oxygen of the gas mix exceeds a safe limit...
of the rebreather. It is important that the diluent is not an oxygen-free gas, such as pure nitrogen or helium, and is breathable; it may be used in an emergency either to flush the loop with breathable gas or as a bailout.
Carbon dioxide scrubber
The exhaled gases are directed through the chemical scrubber, a canister full of some suitable carbon dioxide absorbent such as a form of soda limeSoda lime
Soda lime is a mixture of chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to prevent CO2 retention and carbon dioxide poisoning.It is made by treating...
, which removes the carbon dioxide from the gas mixture and leaves the oxygen and other gases available for re-breathing.
Some absorbent chemical designed for diving applications are Sofnolime, Drager
Dräger
The Drägerwerk AG is a German company based in Lübeck which makes breathing and protection equipment, gas detection and analysis systems, and noninvasive patient monitoring technologies. Customers include hospitals, fire departments and diving companies....
sorb, or Sodasorb. Some systems use a prepackaged Reactive Plastic Curtain (RPC) based cartridge: Reactive Plastic Curtain (RPC) was first used between Micropore Inc. and the US Navy to describe Micropore's absorbent curtains for emergency submarine use, and then more recently RPC has been used on the web to describe their Reactive Plastic Cartridges – ExtendAir.
The carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
passing through the scrubber absorbent is removed when it reacts with the absorbent in the canister; this chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
is exothermic
Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
. This reaction occurs along a "front" which is a cross section of the canister, of the unreacted soda lime that is exposed to carbon dioxide-laden gas. This front moves through the scrubber canister, from the gas input end to the gas output end, as the reaction consumes the active ingredients. However, this front would be a wide zone, because the carbon dioxide in the gas going through the canister needs time to reach the surface of a grain of absorbent, and then time to penetrate to the middle of each grain of absorbent as the outside of the grain becomes exhausted.
In larger environments, such as recompression chambers, a fan is used to pass gas through the canister.
Scrubber failure
The term "break through" means the failure of the "scrubber" to continue removing carbon dioxide from the exhaled gas mix.There are several ways that the scrubber may fail or become less efficient:
- Complete consumption of the active ingredient ("break through").
- The scrubber canister has been incorrectly packed or configured. This allows the exhaled gas to bypass the absorbent. In a rebreather, the soda lime must be packed tightly so that all exhaled gas comes into close contact with the granules of soda lime and the loop is designed to avoid any spaces or gaps between the soda lime and the loop walls that would let gas avoid contact with the absorbent. If any of the seals, such as o rings, or spacers that prevent bypassing of the scrubber, are not cleaned or lubricated or fitted properly, the scrubber will be less efficient, or outside water or gas may get in circuit.
- When the gas mix is under pressure caused by depth, the inside of the canister is more crowded by other gas molecules (oxygen or diluent) and the carbon dioxide molecules are not so free to move around to reach the absorbent. In deep diving with a nitrox or other gas-mixture rebreather, the scrubber needs to be bigger than is needed for a shallow-water or industrial oxygen rebreather, because of this effect. Among British naval rebreather divers, this type of carbon dioxide poisoning was called shallow water blackoutShallow water blackoutA shallow water blackout is a loss of consciousness caused by cerebral hypoxia towards the end of a breath-hold dive in water typically shallower than five metres , when the swimmer does not necessarily experience an urgent need to breathe and has no other obvious medical condition that might have...
. - A Caustic Cocktail – Soda lime is caustic and can cause burns to the eyes and skin. A "caustic cocktail" is a mixture of water and soda lime that occurs when the "scrubber" floods. It gives rise to a chalky taste, which should prompt the diver to switch to an alternative source of breathing gasBreathing gasBreathing gas is a mixture of gaseous chemical elements and compounds used for respiration.Air is the most common and only natural breathing gas...
and rinse his or her mouth out with water. Many modern diving rebreather absorbents are designed not to produce "cocktail" if they get wet. - in below-freezing operation (primarily mountain climbing) the wet scrubber chemicals can freeze when oxygen bottles are changed, thus preventing CO2 from reaching the scrubber material.
Failure prevention
- An indicating dyeDyeA dye is a colored substance that has an affinity to the substrate to which it is being applied. The dye is generally applied in an aqueous solution, and requires a mordant to improve the fastness of the dye on the fiber....
in the soda lime. It changes the colour of the soda lime after the active ingredient is consumed. For example, a rebreather absorbent called "Protosorb" supplied by Siebe GormanSiebe GormanSiebe Gorman & Company Ltd was a British company which developed diving equipment and breathing equipment and worked on commercial diving and marine salvage projects...
had a red dye, which was said to go white when the absorbent was exhausted. Color indicating dye was removed from US Navy fleet use in 1996 when it was suspected of releasing chemicals into the circuit. With a transparent canister, this may be able to show the position of the reaction "front". This is useful in dry open environments, but is not useful on diving equipment, where:- A transparent canister would likely be brittle and easily cracked by knocks.
- Opening the canister to look inside would flood it with water or let unbreathable external gas in.
- The canister is usually out of sight of the user, e.g. inside the breathing bag or inside a backpack box.
- Temperature monitoring. As the reaction between carbon dioxide and soda lime is exothermic, temperature sensors, most likely digital, along the length of the scrubber can be used to measure the position of the front and therefore the life of the scrubber. http://www.apdiving.com/rebreathers/vision/scrubbermonitor/
- Diver training. Divers are trained to monitor and plan the exposure time of the soda lime in the scrubber and replace it within the recommended time limit. At present, there is no effective technology for detecting the end of the life of the scrubber or a dangerous increase in the concentration of carbon dioxide causing carbon dioxide poisoning. The diver must monitor the exposure of the scrubber and replace it when necessary.
- Carbon dioxide gas sensors exist, the first detector to be produced for rebreathers in a diving application was patented by Clive Wilcox of Amphilogic. Such systems are not useful as a tool for monitoring scrubber life when underwater as the onset of scrubber "break through" occurs quite rapidly. Such systems should be used as an essential safety device to warn divers to bail off the loop immediately.
Effectiveness
In rebreather diving, the typical effective duration of the scrubber will be half an hour to several hours of breathing, depending on the granularity and composition of the soda lime, the ambient temperature, the design of the rebreather, and the size of the canister. In some dry open environments, such as a recompression chamber or a hospital, it may be possible to put fresh absorbent in the canister when break through occurs.Controlling the mix
A basic need with a rebreather is to keep the partial pressurePartial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of oxygen (ppO2) in the mix from getting too low (causing hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
) or too high (causing oxygen toxicity
Oxygen toxicity
Oxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen at elevated partial pressures. It is also known as oxygen toxicity syndrome, oxygen intoxication, and oxygen poisoning...
). If not enough new oxygen is being added, the proportion of oxygen in the loop may be too low to support life. In humans, the urge to breathe is normally caused by a build-up of carbon dioxide in the blood, rather than lack of oxygen. The resulting serious hypoxia causes sudden blackout with little or no warning. This makes hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
a deadly problem for rebreather divers.
In many rebreathers the diver can control the gas mix and volume in the loop manually by injecting each of the different available gases to the loop and by venting the loop. The loop often has a pressure relief valve to prevent over-pressure injuries caused by over-pressure of the loop.

Electro-galvanic fuel cell
An electro-galvanic fuel cell is an electrical device, one form of which is commonly used to measure the concentration of oxygen gas in scuba diving and medical equipment....
sensors and onboard electronics, which monitor the ppO2, injecting more oxygen if necessary or issuing an audible warning to the diver if the ppO2 reaches dangerously high or low levels.
Counterlung
The counterlung is a flexible part of the loop, which is designed to change in size by the same volume as the diver's lungs when breathing. Its purpose is to let the loop expand to hold the gas exhaled by the diver and to contract when the diver inhales letting the total volume of gas in the lungs and the loop remain constant throughout the diver's breathing cycle.Underwater, the position of the breathing bag, on the chest, over the shoulders, or on the back, has an effect on the ease of breathing. This is due to the pressure difference between the counterlung and the diver's lung caused by the vertical distance between the two. It is easier to inhale from a front mounted counterlung and exhale to a back mounted counterlung for diver swimming facedown and horizontally.
The design of the rebreathers' counterlungs can also affect the swimming diver's streamlining
Streamline (swimming)
Used most typically in competitive swimming, the streamline position is the position a swimmer takes underwater after pushing off a pool wall. To streamline, a swimmer must tuck the head into the collar bone, pointing both arms straight ahead in a tight line. The underside of both arms should be...
due to location of the counterlungs themselves. Some are designed as over-the-shoulder lungs (e.g. Innerspace Systems Megalodon), while others incorporate the counter lungs into a solid case (e.g. The KISS Classic).
For use out of water, this does not matter so much: for example, in an industrial version of the Siebe Gorman Salvus
Siebe Gorman Salvus
The Siebe Gorman Salvus is a light oxygen rebreather for industrial use or in shallow diving. Its duration on a filling is 30 to 40 minutes. It was very common in Britain during World War II and for a long time afterwards...
the breathing bag hangs down by the left hip.
A rebreather whose counterlung is rubber
Rubber
Natural rubber, also called India rubber or caoutchouc, is an elastomer that was originally derived from latex, a milky colloid produced by some plants. The plants would be ‘tapped’, that is, an incision made into the bark of the tree and the sticky, milk colored latex sap collected and refined...
and not in an enclosed casing, should be sheltered from sunlight
Sunlight
Sunlight, in the broad sense, is the total frequency spectrum of electromagnetic radiation given off by the Sun. On Earth, sunlight is filtered through the Earth's atmosphere, and solar radiation is obvious as daylight when the Sun is above the horizon.When the direct solar radiation is not blocked...
when not in use, to prevent the rubber from perishing due to UV light.
Bailout
While the diver is underwater, the rebreather may fail and be unable to provide a safe breathing mix for the duration of the ascent back to the surface. In this case the diver needs an alternative breathing source: the bailout.Although some rebreather divers—referred to as "alpinists"—do not carry bailouts, bailout strategy becomes a crucial part of dive planning, particularly for long dives and deeper dives in technical diving
Technical diving
Technical diving is a form of scuba diving that exceeds the scope of recreational diving...
. Often the planned dive is limited by the capacity of the bailout and not the capacity of the rebreather.
Several types of bailout are possible:
- An open-circuit demand valve connected to the rebreather's diluent cylinder. While this option has the advantages of being permanently mounted on the rebreather and not heavy, the quantity of gas held by the rebreather is small so the protection offered is low.
- An open-circuit demand valve connected to the rebreather's oxygen cylinder. This is similar to the open circuit diluent bailout except it can only safely be used in depths of 6 metres (19.7 ft) or less because of the risk of oxygen toxicity.
- An independent open-circuit system. The extra cylinders are heavy and cumbersome but larger cylinders let the diver carry more gas providing protection for the ascent from deeper and long dives. The breathing gasBreathing gasBreathing gas is a mixture of gaseous chemical elements and compounds used for respiration.Air is the most common and only natural breathing gas...
mix must be carefully chosen to be safe at all depths of the ascent. - An independent closed-circuit system.
Casing
Many rebreathers have their main parts in a hard backpack casing. This casing needs venting to let surrounding water or air in and out to allow for volume changes as the breathing bag inflates and deflates. In a diving rebreather this needs fairly large holes, including a hole at the bottom to drain the water out when the diver comes out of water. The SEFASEFA
The SEFA is a make of backpack industrial breathing set formerly made by Sabre Safety. It is an oxygen rebreather. "SEFA" is an acronym for "Selected Elevated Flow Apparatus".-Description:...
, which is used for mine rescue
Mine rescue
Mine rescue is the very specialized job of rescuing miners and others who have become trapped or injured underground in mines because of mining accidents and disasters such as explosions caused by firedamp, roof falls or floods.- Expert volunteers :...
, to keep grit and stones out of its working, is completely sealed, except for a large vent panel covered with metal mesh
Mesh
Mesh consists of semi-permeable barrier made of connected strands of metal, fiber, or other flexible/ductile material. Mesh is similar to web or net in that it has many attached or woven strands.-Types of mesh:...
, and holes for the oxygen cylinder's on/off valve and the cylinder pressure gauge. Underwater the casing also serves for streamlining
Streamline (swimming)
Used most typically in competitive swimming, the streamline position is the position a swimmer takes underwater after pushing off a pool wall. To streamline, a swimmer must tuck the head into the collar bone, pointing both arms straight ahead in a tight line. The underside of both arms should be...
, e.g. in the IDA71 and Cis-Lunar
Cis-Lunar
Cis-Lunar is a firm that made computer-controlled closed-circuit automatic rebreathers for scuba diving. Some of their production models were in a streamlined casing. The firm's first plan was to develop spacesuit kit. The dot.com crash in early 2000 prevented Cis-Lunar from financing mass...
.
Diffuser
Some military rebreathers have a diffuser over the blowoff valve, which helps to conceal the diver's presence by masking the release of bubbles.Arrangement
The parts of a rebreather can be arranged on the wearer's body in many ways. For example:- In this early Russian Epron-1 rebreather, the canister and the breathing bag and the oxygen cylinder are each vertical on the chest, in order left to right; the breathing tube loop goes from the end of the canister to the bag.
- In this old German industrial rebreather, the working parts are on the user's left waist and it has one long breathing tube.
Risks
The percentage of deaths that involve the use of a rebreather among United States and Canadian residents increased from approximately 1 to 5% of the total diving fatalities collected by the Divers Alert NetworkDivers Alert Network
The Divers Alert Network is a non-profit 501 organization devoted to assisting divers in need. The Research department conducts significant medical research on recreational scuba diving safety...
from 1998 through 2004. Investigations into rebreather deaths focus on three main areas: medical, equipment, and procedural.
In mountaineering, closed-circuit rebreathers are ideal to treat various altitude related illnesses as the user is brought back to sea level in terms of oxygen partial pressure (pp). The danger is that a sick climber using a rebreather might become unconscious. Because an absolute atmospheric seal is required for rebreathers to work correctly, such a seal could conceivably cause an unconscious user to suffocate when the oxygen ran out or the scrubber became exhausted. (Because there has been very little use of mountaineering rebreathers, this danger is still only theoretical.)
Closed circuit disorders
In addition to the other diving disorders suffered by divers, rebreather divers are also more susceptible to the following disorders (all of which are directly connected with the effectiveness of actual rebreather designs and construction, not with the theory of rebreathing):- Sudden blackout due to hypoxia caused by too low a partial pressurePartial pressureIn a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of oxygen in the loop. A particular problem when using a closed circuit rebreather is the drop in ambient pressure caused by the ascent phase of the dive, which reduces the partial pressure of oxygen to hypoxic levels leading to what is sometimes called deep water blackoutDeep water blackoutA deep water blackout is a loss of consciousness caused by cerebral hypoxia on ascending from a deep freedive or breath-hold dive, typically of ten metres or more when the swimmer does not necessarily experience an urgent need to breathe and has no other obvious medical condition that might have...
. - SeizureSeizureAn epileptic seizure, occasionally referred to as a fit, is defined as a transient symptom of "abnormal excessive or synchronous neuronal activity in the brain". The outward effect can be as dramatic as a wild thrashing movement or as mild as a brief loss of awareness...
s due to oxygen toxicityOxygen toxicityOxygen toxicity is a condition resulting from the harmful effects of breathing molecular oxygen at elevated partial pressures. It is also known as oxygen toxicity syndrome, oxygen intoxication, and oxygen poisoning...
caused by too high a partial pressure of oxygen in the loop. This can be caused by the rise in ambient pressure caused by the descent phase of the dive, which raises the partial pressure of oxygen to hyperoxic levels. In fully closed circuit equipment, aging oxygen sensorsElectro-galvanic fuel cellAn electro-galvanic fuel cell is an electrical device, one form of which is commonly used to measure the concentration of oxygen gas in scuba diving and medical equipment....
may become "current limited" and fail to measure high partial pressures of oxygen resulting in dangerously high oxygen levels. - Disorientation, panicPanicPanic is a sudden sensation of fear which is so strong as to dominate or prevent reason and logical thinking, replacing it with overwhelming feelings of anxiety and frantic agitation consistent with an animalistic fight-or-flight reaction...
, headacheHeadacheA headache or cephalalgia is pain anywhere in the region of the head or neck. It can be a symptom of a number of different conditions of the head and neck. The brain tissue itself is not sensitive to pain because it lacks pain receptors. Rather, the pain is caused by disturbance of the...
, and hyperventilationHyperventilationHyperventilation or overbreathing is the state of breathing faster or deeper than normal, causing excessive expulsion of circulating carbon dioxide. It can result from a psychological state such as a panic attack, from a physiological condition such as metabolic acidosis, can be brought about by...
due to excess of carbon dioxideHypercapniaHypercapnia or hypercapnea , also known as hypercarbia, is a condition where there is too much carbon dioxide in the blood...
caused by incorrect configuration, failure or inefficiency of the scrubberSoda limeSoda lime is a mixture of chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to prevent CO2 retention and carbon dioxide poisoning.It is made by treating...
. The scrubber must be configured so that no exhaled gas can bypass it; it must be packed and sealed correctly. Another problem is the diver producing carbon dioxide faster than the absorbent can handle; for example, during hard work or fast swimming. The solution to this is to slow down and let the absorbent catch up. The scrubber efficiency may be reduced at depth where the increased concentration of other gas molecules, due to pressure, stops all the carbon dioxide molecules reaching the active ingredient of the scrubber. - The rebreather diver must keep breathing in and out all the time, to keep the exhaled gas flowing over the carbon dioxide absorbent, so the absorbent can work all the time. Divers need to lose any air conservation habits that may have been developed while diving with open-circuit scuba. In closed circuit rebreathers, this also has the advantage of mixing the gases preventing oxygen-rich and oxygen-lean spaces developing within the loop, which may give inaccurate readings to the oxygen control system.
- "Caustic cocktail" in the loop if water comes into contact with the soda limeSoda limeSoda lime is a mixture of chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to prevent CO2 retention and carbon dioxide poisoning.It is made by treating...
used in the carbon dioxideCarbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
scrubber. The diver is normally alerted to this by a chalky taste in the mouth. A safe response is to bail out to "open circuit" and rinse the mouth out.
Restoring the oxygen content of the loop
Many diver training organizations teach the "diluent flush" technique as a safe way to restore the mix in the loop to a level of oxygen that is neither too high nor too low. It only works when partial pressurePartial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of oxygen in the diluent alone would not cause hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
or hyperoxia
Hyperoxia
Hyperoxia is excess oxygen or higher than normal partial pressure of oxygen.In medicine, it refers to excess oxygen in the lungs or other body tissues, which can be caused by breathing air or oxygen at pressures greater than normal atmospheric pressure...
, such as when using a normoxic diluent and observing the diluent's maximum operating depth
Maximum operating depth
In technical diving and nitrox diving, the maximum operating depth of a breathing gas is the depth at which the partial pressure of oxygen of the gas mix exceeds a safe limit...
. The technique involves simultaneously venting the loop and injecting diluent. This flushes out the old mix and replaces it with a known proportion of oxygen
Compared with open circuit
When compared with Aqua-LungAqua-lung
Aqua-Lung was the original name of the first open-circuit free-swimming underwater breathing set in reaching worldwide popularity and commercial success...
s, rebreathers have some disadvantages including expense, complexity of operation and maintenance, and fewer failsafes. A malfunctioning rebreather can supply a gas mixture which contains too little oxygen to sustain life, or it may allow carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
to build up to dangerous levels. Typically rebreathers try to solve these problems by monitoring the system with electronics, sensors and alarm systems. These are expensive and susceptible to failure, improper configuration and misuse.
The bailout requirement of rebreather diving can sometimes also require a rebreather diver to carry almost as much bulk of cylinders
Diving cylinder
A diving cylinder, scuba tank or diving tank is a gas cylinder used to store and transport high pressure breathing gas as a component of a scuba set. It provides gas to the scuba diver through the demand valve of a diving regulator....
as an open-circuit diver so the diver can complete the necessary decompression stops if the rebreather fails completely. Some rebreather divers prefer not to carry enough bailout for a safe ascent breathing open circuit, but instead rely on the rebreather, believing that an irrecoverable rebreather failure is very unlikely. This practice is known as alpinism or alpinist diving and is generally maligned due to the perceived extremely high risk of death if the rebreather fails.
Sport diving rebreather technology innovations
Over the past ten or fifteen years rebreather technology has advanced considerably, often driven by the growing market in recreational diving equipment. Innovations include:- The electronic, fully closed circuit rebreather itself – use of electronics and electro-galvanic fuel cellElectro-galvanic fuel cellAn electro-galvanic fuel cell is an electrical device, one form of which is commonly used to measure the concentration of oxygen gas in scuba diving and medical equipment....
s to monitor oxygen concentration within the loop and maintain a certain partial pressurePartial pressureIn a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
of oxygen - Automatic diluent valves – these inject diluent gas into the loop when the loop pressure falls below the limit at which the diver can comfortably breathe.
- Dive/surface valves or bailout valves – a device in the mouthpiece on the loop which connects to a bailout demand valve and can be switched to provide gas from either the loop or the demand valve without the diver taking the mouthpiece from his or her mouth. An important safety device when carbon dioxide poisoning occurs.
- Integrated decompression computers – these allow divers to take advantage of the content and generate a schedule of decompression stops.
- Carbon dioxide scrubber life monitoring systems – temperature sensors monitor the progress of the reaction of the soda limeSoda limeSoda lime is a mixture of chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to prevent CO2 retention and carbon dioxide poisoning.It is made by treating...
and provide an indication of when the scrubber will be exhausted. - Carbon dioxide monitoring systems – Gas sensing cell and interpretive electronics which detect the presence of carbon dioxide in the unique environment of a rebreather loop. The first ever system that was proved to function correctly was patented by Clive Wilcox of Amphilogic.
See also
- Escape setEscape setAn escape set is a breathing set, which lets its wearer survive for a time in an environment without breathable air, in particular underwater, primarily or originally intending mainly to survive long enough to reach safety where the air is breathable.The escape set was developed from such...
- SCBA (surface-only (industrial) rebreathers)
- Portable Life Support System
- NIOSH Docket # 123, titled "Reevaluation of NIOSH limitations on and precaution for safe use of positive-pressure closed-circuit SCBA" is available at the link http://www.cdc.gov/niosh/review/public/123/default.html
- CDLSECDLSEThe CDLSE is made by Divex in Aberdeen, Scotland.It is an electronic closed circuit rebreather designed to be silent and non-magnetic...
Clearance Divers' Life Support Equipment. - FROGSFROGS (rebreather)FROGS is a make of rebreather for frogmen using oxygen, which has been used by the French Navy and the Commando Hubert since 15 October 2002. It is made by the diving gear manufacturers Aqualung....
Full Range Oxygen Gas System. - KISS rebreatherKISS (rebreather)KISS is a type of manually operated closed circuit diving rebreather designed on the KISS principle.It was designed by Gordon Smith of Jetsam Technologies.-External links:* – Manufacturer's website...
- David Shaw (diver)David Shaw (diver)David Shaw was an Australian scuba diver, a technical diver and an airline pilot for Cathay Pacific, who flew the A330-300, A340-300 and A340-600...
Information sources
- RebreatherPro Free searchable multimedia resource for rebreather divers
- Rebreather Scuba Diving Rebreather world contains further information on rebreathers. The site includes a Rebreather library and Rebreather Forums, and Rebreather Trips, Vacations, and Holidays.
- Richard Pyle's rebreather page
- The Rebreather Site Information on many makes of rebreathers
- Diver Dave's site. It has many detailed photographs of rebreathers and their components.
- Karl Kramer's site (more history)
- Teknosofen homepage Åke's Rebreather Related Page
- Image gallery of LAR-6 and LAR-7 and FGT II and LAR V rebreathers, and other combat frogman's kit
- Richard Pyle's rebreather page: in-depth explanation on how rebreathers work and many useful references in its "Further Reading" section
- A history of closed circuit oxygen underwater breathing apparatus, published in 1970, plenty of images, including mountaineering rebreathers, may be slow to download
- Information on shallow water blackout

