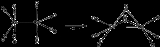
Dicobalt octacarbonyl
Encyclopedia
Dicobalt octacarbonyl is the inorganic compound
Co2(CO)8. This metal carbonyl
is a reagent
and catalyst in organometallic chemistry
and organic synthesis
. It is used as a catalyst for hydroformylation
, the conversion of alkene
s to aldehyde
s.
of cobalt(II) salts, often in the presence of cyanide
. It is an orange-colored, pyrophoric solid that is thermally unstable. It exists as two isomers in solution:
The predominant isomer resembles Fe2(CO)9, less one bridging CO. The Co-Co distance is 2.52 Å, and the Co-COterminal and Co-CObridge distances are 1.80 and 1.90 Å, respectively. These isomer
s rapidly interconvert. The minor isomer has no bridging CO ligands; it is described (CO)4Co-Co(CO)4 (D3d symmetry group
). The major isomer contains two bridging CO ligand and features octahedral
cobalt, describable as (CO)3Co(μ-CO)2Co(CO)3 (C2v symmetry group
). The minor isomer has been crystallized together with C60.
, [HCo(CO)4]:
This hydride is the active agent in hydroformylation. It adds to alkene
s to give an alkyl-Co(CO)4 product that then proceeds to insert CO and undergo hydrogenolysis to produce the aldehyde
. Reduction of Co2(CO)8 gives the conjugate base of HCo(CO)4:
The CO ligands can be replaced with tertiary phosphine
ligand
s to give Co2(CO)8-x(PR3)x. These bulky derivatives are more selective catalysts for hydroformylation reactions. "Hard
" Lewis bases, e.g. pyridine
, cause disproportionation
:
Co2(CO)8 catalyzes the Pauson–Khand reaction
of an alkyne
, an alkene
, and CO to give cyclopentenones.
Heating causes decarbonylation and formation of the tetrahedral cluster:
upon decomposition.
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
Co2(CO)8. This metal carbonyl
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
is a reagent
Reagent
A reagent is a "substance or compound that is added to a system in order to bring about a chemical reaction, or added to see if a reaction occurs." Although the terms reactant and reagent are often used interchangeably, a reactant is less specifically a "substance that is consumed in the course of...
and catalyst in organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
and organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. It is used as a catalyst for hydroformylation
Hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
, the conversion of alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s to aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s.
Synthesis, structure, properties
It is synthesised by the high pressure carbonylationCarbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry.-Organic chemistry:...
of cobalt(II) salts, often in the presence of cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
. It is an orange-colored, pyrophoric solid that is thermally unstable. It exists as two isomers in solution:
The predominant isomer resembles Fe2(CO)9, less one bridging CO. The Co-Co distance is 2.52 Å, and the Co-COterminal and Co-CObridge distances are 1.80 and 1.90 Å, respectively. These isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s rapidly interconvert. The minor isomer has no bridging CO ligands; it is described (CO)4Co-Co(CO)4 (D3d symmetry group
Symmetry group
The symmetry group of an object is the group of all isometries under which it is invariant with composition as the operation...
). The major isomer contains two bridging CO ligand and features octahedral
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
cobalt, describable as (CO)3Co(μ-CO)2Co(CO)3 (C2v symmetry group
Symmetry group
The symmetry group of an object is the group of all isometries under which it is invariant with composition as the operation...
). The minor isomer has been crystallized together with C60.
Reactions
The most characteristic reaction of Co2(CO)8 is its hydrogenation to tetracarbonylhydrocobaltTetracarbonylhydrocobalt
Cobalt tetracarbonyl hydride is the organometallic compound with the formula HCo4. It is a yellow liquid that forms a colorless vapor and has an intolerable odor. Its main use is as a catalyst in hydroformylation.-Structure and properties:...
, [HCo(CO)4]:
- Co2(CO)8 + H2 → 2 HCo(CO)4
This hydride is the active agent in hydroformylation. It adds to alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
s to give an alkyl-Co(CO)4 product that then proceeds to insert CO and undergo hydrogenolysis to produce the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
. Reduction of Co2(CO)8 gives the conjugate base of HCo(CO)4:
- Co2(CO)8 + 2 Na → 2 NaCo(CO)4
The CO ligands can be replaced with tertiary phosphine
Phosphine
Phosphine is the compound with the chemical formula PH3. It is a colorless, flammable, toxic gas. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odor like garlic or rotting fish, due to the presence of substituted phosphine and diphosphine...
ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
s to give Co2(CO)8-x(PR3)x. These bulky derivatives are more selective catalysts for hydroformylation reactions. "Hard
HSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
" Lewis bases, e.g. pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, cause disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
:
- 6 C6H5N + 1.5 Co2(CO)8 → [Co(C6H5N)6][Co(CO)4]2 + 4 CO
Co2(CO)8 catalyzes the Pauson–Khand reaction
Pauson–Khand reaction
The Pauson–Khand reaction is a chemical reaction described as a [2+2+1] cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone...
of an alkyne
Alkyne
Alkynes are hydrocarbons that have a triple bond between two carbon atoms, with the formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature...
, an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
, and CO to give cyclopentenones.
Heating causes decarbonylation and formation of the tetrahedral cluster:
- 2 Co2(CO)8 → Co4(CO)12 + 4 CO
Safety
Co2(CO)8 a volatile source of cobalt(0), is pyrophoric and releases carbon monoxideCarbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
upon decomposition.


