Isomer
Encyclopedia
In chemistry
, isomers (from Greek ἰσομερής, isomerès; isos = "equal", méros = "part") are compounds with the same molecular formula but different structural formula
s. Isomers do not necessarily share similar properties, unless they also have the same functional group
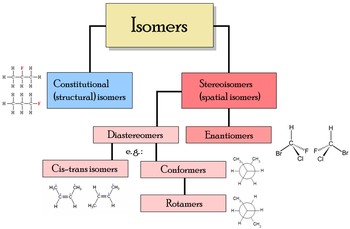
s. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical isomers, etc. (see chart below). There are two main forms of isomerism: structural isomerism
and stereoisomerism
(spatial isomerism).
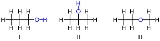
 In structural isomers, sometimes referred to as constitutional isomers, the atoms and functional group
In structural isomers, sometimes referred to as constitutional isomers, the atoms and functional group
s are joined together in different ways. Structural isomers have different IUPAC names and may or may not belong to the same functional group. This group includes chain isomerism whereby hydrocarbon
chains have variable amounts of branching; position isomerism which deals with the position of a functional group on a chain; and functional group isomerism in which one functional group is split up into different ones.
For example, two position isomers would be 2-fluoropropane and 1-fluoropropane, illustrated on the right.
In skeletal isomers the main carbon chain is different between the two isomers. This type of isomerism is most identifiable in secondary and tertiary alcohol isomers.
Tautomer
s are structural isomers of the same chemical substance that spontaneously interconvert with each other, even when pure. They have different chemical properties, and consequently, distinct reactions characteristic to each form are observed. If the interconversion reaction is fast enough, tautomers cannot be isolated from each other. An example is when they differ by the position of a proton, such as in keto/enol tautomerism, where the proton is alternately on the carbon or oxygen.
s where different isomers are non-superimposable mirror-images of each other, and diastereomer
s when they are not.
Diastereomerism is again subdivided into "cis-trans isomers", which have restricted rotation within the molecule (typically isomers containing a double bond) and "conformational isomers"
(conformers), which can rotate about one or more single bonds within the molecule.
An obsolete term for "cis-trans isomerism" is "geometric isomers".
For compounds with more than two substituents E-Z notation
is used instead of cis and trans. If possible, E and Z (written in italic type
) is also preferred in compounds with two substituents.
In octahedral
coordination compounds, facial-meridional isomerism occurs. The isomers can be fac- (with facial ligands) or mer- (with meridional ligands).
Note that although conformers can be referred to as stereoisomers, they are not stable isomers, since bonds in conformers can easily rotate thus converting one conformer to another which can be either diastereomeric or enantiomeric to the original one.
While structural isomers typically have different chemical properties, stereoisomers behave identically in most chemical reactions, except in their reaction with other stereoisomers. Enzyme
s however can distinguish between different enantiomers of a compound, and organisms often prefer one isomer over the other. Some stereoisomers also differ in the way they rotate polarized light.
, and so exist in roughly equal amounts, provided that they can interconvert relatively freely, that is the energy barrier between the two isomers is not too high. When the isomerisation occurs intramolecular
ly it is considered a rearrangement reaction
.
An example of an organometallic
isomerisation is the production of decaphenylferrocene, [(η5-C5Ph5)2Fe] from its linkage isomer.
The energy difference between two isomers is called isomerisation energy. Isomerisations with low energy difference both experimental and computational (in parentheses) are endothermic
trans-cis isomerisation of 2-butene
with 2.6 (1.2) kcal
/mol
, cracking of isopentane
to n-pentane with 3.6 (4.0) kcal/mol or conversion of trans-2-butene to 1-butene
with 2.6 (2.4) kcal/mol.
: it has the formula C
3H
8O
(or C3H7OH
) and occurs as two isomers: propan-1-ol
(n-propyl alcohol; I) and propan-2-ol (isopropyl alcohol; II)
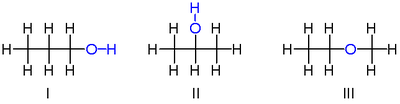 Note that the position of the oxygen
Note that the position of the oxygen
atom differs between the two: it is attached to an end carbon
in the first isomer, and to the center carbon in the second.
There is, however, another isomer of C
3H
8O
which has significantly different properties: methoxyethane
(methyl-ethyl-ether; III). Unlike the isomers of propanol, methoxyethane has an oxygen connected to two carbons rather than to one carbon and one hydrogen. This makes it an ether
, not an alcohol
, as it lacks a hydroxyl group, and has chemical properties more similar to other ethers than to either of the above alcohol isomers.
Examples of isomers having different medical properties can be easily found. For example, in the placement of methyl group
s. In substituted xanthine
s, Theobromine
, found in chocolate
, is a vasodilator with some effects in common with caffeine
, but if one of the two methyl groups is moved to a different position on the two-ring core, the isomer is theophylline
, which has a variety of effects, including bronchodilation
and anti-inflammatory
action. Another example of this occurs in the phenethylamine
-based stimulant drugs. Phentermine
is a non-chiral
compound with a weaker effect than amphetamine
. It is used as an appetite reducing medication and has mild or no stimulant properties. However, a different atomic arrangement gives dextromethamphetamine which is a stronger stimulant than amphetamine.
Allene
and propyne
are examples of isomers containing different bond types. Allene contains two double bonds, whereas propyne contains one triple bond
.
proceeds via the cis-trans isomerization of maleic acid
:
In medicinal chemistry and biochemistry, enantiomer
s are a special concern because they may possess quite different biological activity. The infamous case of thalidomide
arose from the effects of the unwanted enantiomer. Many preparative procedure afford a mixture of equal amounts of both enantiomeric forms. In some cases, the enantiomers are separated by chromatography
using chiral stationary phases. In other cases, enantioselective syntheses have been developed.
(prepared by Justus von Liebig
the previous year), its properties were quite different. This finding challenged the prevailing chemical understanding of the time, which held that chemical compound
s could be different only when they had different elemental compositions. After additional discoveries of the same sort were made, such as Woehler's 1828 discovery that urea
had the same atomic composition as the chemically distinct ammonium cyanate, Jöns Jakob Berzelius
introduced the term isomerism in 1830 to describe the phenomenon.
In 1848, Louis Pasteur
separated tiny crystals of tartaric acid
into their two mirror-image
forms. The individual molecules of each were the left and right optical stereoisomers, solutions of which rotate the plane of polarized light
to the same degree but in opposite directions.
s are generally large molecules that wind about and form different shaped knots or loops. Molecules with topoisomers include catenane
s and DNA
. Topoisomerase
enzymes can knot DNA and thus change its topology. There are also isotopomers
or isotopic
isomers that have the same numbers of each type of isotopic substitution but in chemically different positions. In nuclear physics
, nuclear isomer
s are excited states of atomic nuclei. Spin isomers
have differing distributions of spin
among their constituent atoms.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, isomers (from Greek ἰσομερής, isomerès; isos = "equal", méros = "part") are compounds with the same molecular formula but different structural formula
Structural formula
The structural formula of a chemical compound is a graphical representation of the molecular structure, showing how the atoms are arranged. The chemical bonding within the molecule is also shown, either explicitly or implicitly...
s. Isomers do not necessarily share similar properties, unless they also have the same functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical isomers, etc. (see chart below). There are two main forms of isomerism: structural isomerism
Structural isomerism
Structural isomerism, or constitutional isomerism , is a form of isomerism in which molecules with the same molecular formula have bonded together in different orders, as opposed to stereoisomerism. There are multiple synonyms for constitutional isomers.Three categories of constitutional isomers...
and stereoisomerism
Stereoisomerism
Stereoisomers are isomeric molecules that have the same molecular formula and sequence of bonded atoms , but that differ only in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections...
(spatial isomerism).
Structural isomers

Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s are joined together in different ways. Structural isomers have different IUPAC names and may or may not belong to the same functional group. This group includes chain isomerism whereby hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
chains have variable amounts of branching; position isomerism which deals with the position of a functional group on a chain; and functional group isomerism in which one functional group is split up into different ones.
For example, two position isomers would be 2-fluoropropane and 1-fluoropropane, illustrated on the right.
In skeletal isomers the main carbon chain is different between the two isomers. This type of isomerism is most identifiable in secondary and tertiary alcohol isomers.
Tautomer
Tautomer
Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. This reaction commonly results in the formal migration of a hydrogen atom or proton, accompanied by a switch of a single bond and adjacent double bond...
s are structural isomers of the same chemical substance that spontaneously interconvert with each other, even when pure. They have different chemical properties, and consequently, distinct reactions characteristic to each form are observed. If the interconversion reaction is fast enough, tautomers cannot be isolated from each other. An example is when they differ by the position of a proton, such as in keto/enol tautomerism, where the proton is alternately on the carbon or oxygen.
Stereoisomers
In stereoisomers the bond structure is the same, but the geometrical positioning of atoms and functional groups in space differs. This class includes enantiomerEnantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s where different isomers are non-superimposable mirror-images of each other, and diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s when they are not.
Diastereomerism is again subdivided into "cis-trans isomers", which have restricted rotation within the molecule (typically isomers containing a double bond) and "conformational isomers"
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
(conformers), which can rotate about one or more single bonds within the molecule.
An obsolete term for "cis-trans isomerism" is "geometric isomers".
For compounds with more than two substituents E-Z notation
E-Z notation
E-Z notation, or the E-Z convention, is the IUPAC preferred method of describing the stereochemistry of double bonds in organic chemistry...
is used instead of cis and trans. If possible, E and Z (written in italic type
Italic type
In typography, italic type is a cursive typeface based on a stylized form of calligraphic handwriting. Owing to the influence from calligraphy, such typefaces often slant slightly to the right. Different glyph shapes from roman type are also usually used—another influence from calligraphy...
) is also preferred in compounds with two substituents.
In octahedral
Octahedral molecular geometry
In chemistry, octahedral molecular geometry describes the shape of compounds where in six atoms or groups of atoms or ligands are symmetrically arranged around a central atom, defining the vertices of an octahedron...
coordination compounds, facial-meridional isomerism occurs. The isomers can be fac- (with facial ligands) or mer- (with meridional ligands).
Note that although conformers can be referred to as stereoisomers, they are not stable isomers, since bonds in conformers can easily rotate thus converting one conformer to another which can be either diastereomeric or enantiomeric to the original one.
While structural isomers typically have different chemical properties, stereoisomers behave identically in most chemical reactions, except in their reaction with other stereoisomers. Enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s however can distinguish between different enantiomers of a compound, and organisms often prefer one isomer over the other. Some stereoisomers also differ in the way they rotate polarized light.
Isomerisation
Isomerisation is the process by which one molecule is transformed into another molecule which has exactly the same atoms, but the atoms are rearranged. In some molecules and under some conditions, isomerisation occurs spontaneously. Many isomers are equal or roughly equal in bond energyBond energy
In chemistry, bond energy is the measure of bond strength in a chemical bond. It is the heat required to break one Mole of molecules into their individual atoms. For example, the carbon-hydrogen bond energy in methane E is the enthalpy change involved with breaking up one molecule of methane into...
, and so exist in roughly equal amounts, provided that they can interconvert relatively freely, that is the energy barrier between the two isomers is not too high. When the isomerisation occurs intramolecular
Intramolecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.- Examples :...
ly it is considered a rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
.
An example of an organometallic
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
isomerisation is the production of decaphenylferrocene, [(η5-C5Ph5)2Fe] from its linkage isomer.
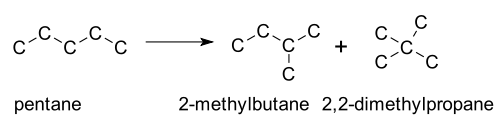
Instances of Isomerization
- Isomerizations in hydrocarbon crackingCracking (chemistry)In petroleum geology and chemistry, cracking is the process whereby complex organic molecules such as kerogens or heavy hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products...
. This is usually employed in organic chemistryOrganic chemistryOrganic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, where fuels, such as pentanePentanePentane is an organic compound with the formula C5H12 — that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer; the other two being called...
, a straight-chain isomer, are heated in the presence of a platinum catalyst. The resulting mixture of straight- and branched-chain isomers then have to be separated. An industrial process is also the isomerisation of n-butane into isobutaneIsobutaneIsobutane, also known as methylpropane, is an isomer of butane. It is the simplest alkane with a tertiary carbon. Concerns with depletion of the ozone layer by freon gases have led to increased use of isobutane as a gas for refrigeration systems, especially in domestic refrigerators and freezers,...
.
-
- Trans-cis isomerism. In certain compounds an interconversion of cis and trans isomersGeometric isomerismIn organic chemistry, cis/trans isomerism or geometric isomerism or configuration isomerism or E/Z isomerism is a form of stereoisomerism describing the orientation of functional groups within a molecule...
can be observed, for instance, with maleic acidMaleic acidMaleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
and with azobenzeneAzobenzeneAzobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
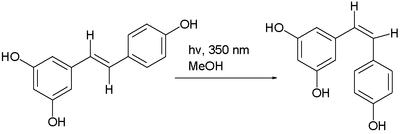
often by photoisomerization. Another example is the photochemical conversion of the trans isomer to the cis isomer of resveratrolResveratrolResveratrol is a stilbenoid, a type of natural phenol, and a phytoalexin produced naturally by several plants when under attack by pathogens such as bacteria or fungi....
:
- Trans-cis isomerism. In certain compounds an interconversion of cis and trans isomers
- Aldose-ketose isomerism in biochemistry.
- Isomerisations between conformational isomers. These take place without an actual rearrangement for instance inconversion of two cyclohexane conformationCyclohexane conformationA cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds....
s - Fluxional molecules display rapid interconversion of isomers e.g. BullvaleneBullvaleneBullvalene is a hydrocarbon with the chemical formula C10H10 with the unusual property that the chemical bonds making up the molecule are constantly rearranging as in fluxional molecules...
. - valence isomerisation: the isomerisation of molecules which involve structural changes resulting only from a relocation of single and double bondDouble bondA double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s. If a dynamic equilibrium is established between the two isomers it is also referred to as valence tautomerism
The energy difference between two isomers is called isomerisation energy. Isomerisations with low energy difference both experimental and computational (in parentheses) are endothermic
Endothermic
In thermodynamics, the word endothermic describes a process or reaction in which the system absorbs energy from the surroundings in the form of heat. Its etymology stems from the prefix endo- and the Greek word thermasi,...
trans-cis isomerisation of 2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
with 2.6 (1.2) kcal
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
/mol
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
, cracking of isopentane
Isopentane
Isopentane, C5H12, also called methylbutane or 2-methylbutane, is a branched-chain alkane with five carbon atoms. Isopentane is an extremely volatile and extremely flammable liquid at room temperature and pressure. The normal boiling point is just a few degrees above room temperature and...
to n-pentane with 3.6 (4.0) kcal/mol or conversion of trans-2-butene to 1-butene
1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
with 2.6 (2.4) kcal/mol.
Propanol
A simple example of isomerism is given by propanolPropanol
Propanol may refer to:*Propan-1-ol, or n-propanol: CH3CH2CH2OH, the most common meaning*Propan-2-ol, or isopropyl alcohol, or isopropanol: 2CHOH...
: it has the formula C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
3H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
8O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
(or C3H7OH
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
) and occurs as two isomers: propan-1-ol
Propan-1-ol
1-Propanol is a primary alcohol with the molecular formula of C3H8O, and a structural formula of CH3CH2CH2OH. It is also known as propan-1-ol, 1-propyl alcohol, n-propyl alcohol, n-propanol, or simply propanol. It is an isomer of isopropanol . It is used as a solvent in the pharmaceutical...
(n-propyl alcohol; I) and propan-2-ol (isopropyl alcohol; II)

Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
atom differs between the two: it is attached to an end carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
in the first isomer, and to the center carbon in the second.
There is, however, another isomer of C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
3H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
8O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
which has significantly different properties: methoxyethane
Methoxyethane
Methoxyethane, also known as ethyl methyl ether, is an ethyl group with a bonded methoxy. Methoxyethane is a colorless gaseous ether with a medicine-like odor. It is extremely flammable, and its inhalation may cause asphyxiation or dizzyness. As a Lewis base, it can react with Lewis acids to form...
(methyl-ethyl-ether; III). Unlike the isomers of propanol, methoxyethane has an oxygen connected to two carbons rather than to one carbon and one hydrogen. This makes it an ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
, not an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
, as it lacks a hydroxyl group, and has chemical properties more similar to other ethers than to either of the above alcohol isomers.
Examples of isomers having different medical properties can be easily found. For example, in the placement of methyl group
Methyl group
Methyl group is a functional group derived from methane, containing one carbon atom bonded to three hydrogen atoms —CH3. The group is often abbreviated Me. Such hydrocarbon groups occur in many organic compounds. The methyl group can be found in three forms: anion, cation and radical. The anion...
s. In substituted xanthine
Xanthine
Xanthine , is a purine base found in most human body tissues and fluids and in other organisms. A number of stimulants are derived from xanthine, including caffeine and theobromine....
s, Theobromine
Theobromine
Theobromine , also known as xantheose, is a bitter alkaloid of the cacao plant, with the chemical formula C7H8N4O2. It is found in chocolate, as well as in a number of other foods, including the leaves of the tea plant, and the kola nut...
, found in chocolate
Chocolate
Chocolate is a raw or processed food produced from the seed of the tropical Theobroma cacao tree. Cacao has been cultivated for at least three millennia in Mexico, Central and South America. Its earliest documented use is around 1100 BC...
, is a vasodilator with some effects in common with caffeine
Caffeine
Caffeine is a bitter, white crystalline xanthine alkaloid that acts as a stimulant drug. Caffeine is found in varying quantities in the seeds, leaves, and fruit of some plants, where it acts as a natural pesticide that paralyzes and kills certain insects feeding on the plants...
, but if one of the two methyl groups is moved to a different position on the two-ring core, the isomer is theophylline
Theophylline
Theophylline, also known as dimethylxanthine, is a methylxanthine drug used in therapy for respiratory diseases such as COPD and asthma under a variety of brand names. Because of its numerous side-effects, the drug is now rarely administered for clinical use. As a member of the xanthine family, it...
, which has a variety of effects, including bronchodilation
Bronchodilator
A bronchodilator is a substance that dilates the bronchi and bronchioles, decreasing resistance in the respiratory airway and increasing airflow to the lungs. Bronchodilators may be endogenous , or they may be medications administered for the treatment of breathing difficulties...
and anti-inflammatory
Anti-inflammatory
Anti-inflammatory refers to the property of a substance or treatment that reduces inflammation. Anti-inflammatory drugs make up about half of analgesics, remedying pain by reducing inflammation as opposed to opioids, which affect the central nervous system....
action. Another example of this occurs in the phenethylamine
Phenethylamine
Phenylethylamine or phenethylamine is a natural monoamine alkaloid, trace amine, and also the name of a class of chemicals with many members well known for psychoactive drug and stimulant effects. Studies suggest that phenylethylamine functions as a neuromodulator or neurotransmitter in the...
-based stimulant drugs. Phentermine
Phentermine
Phentermine, a contraction of "phenyl-tertiary-butylamine", is a psychostimulant drug of the phenethylamine class, chemically related to amphetamine. It is used medically as an appetite suppressant....
is a non-chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
compound with a weaker effect than amphetamine
Amphetamine
Amphetamine or amfetamine is a psychostimulant drug of the phenethylamine class which produces increased wakefulness and focus in association with decreased fatigue and appetite.Brand names of medications that contain, or metabolize into, amphetamine include Adderall, Dexedrine, Dextrostat,...
. It is used as an appetite reducing medication and has mild or no stimulant properties. However, a different atomic arrangement gives dextromethamphetamine which is a stronger stimulant than amphetamine.
Allene
Allene
An allene is a compound in which one carbon atom has double bonds with each of its two adjacent carbon centres. Allenes are classified as polyenes with cumulated dienes. The parent compound of allene is propadiene. Compounds with an allene-type structure but with more than three carbon atoms are...
and propyne
Methylacetylene
Methylacetylene is an alkyne with the chemical formula H3C≡CH. It is a component of MAPP gas along with its isomer 1,2-propadiene , which is commonly used in gas welding...
are examples of isomers containing different bond types. Allene contains two double bonds, whereas propyne contains one triple bond
Triple bond
A triple bond in chemistry is a chemical bond between two chemical elements involving six bonding electrons instead of the usual two in a covalent single bond. The most common triple bond, that between two carbon atoms, can be found in alkynes. Other functional groups containing a triple bond are...
.
Synthesis of fumaric acid
Industrial synthesis of fumaric acidFumaric acid
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans and in maleic acid they are cis...
proceeds via the cis-trans isomerization of maleic acid
Maleic acid
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
:
In medicinal chemistry and biochemistry, enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s are a special concern because they may possess quite different biological activity. The infamous case of thalidomide
Thalidomide
Thalidomide was introduced as a sedative drug in the late 1950s that was typically used to cure morning sickness. In 1961, it was withdrawn due to teratogenicity and neuropathy. There is now a growing clinical interest in thalidomide, and it is introduced as an immunomodulatory agent used...
arose from the effects of the unwanted enantiomer. Many preparative procedure afford a mixture of equal amounts of both enantiomeric forms. In some cases, the enantiomers are separated by chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
using chiral stationary phases. In other cases, enantioselective syntheses have been developed.
History
Isomerism was first noticed in 1827, when Friedrich Woehler prepared cyanic acid and noted that although its elemental composition was identical to fulminic acidFulminic acid
Fulminic acid is a compound with a molecular formula HCNO. This substance was discovered in 1824 by Justus von Liebig. It is an organic acid and an isomer of isocyanic acid, discovered one year later by Friedrich Woehler....
(prepared by Justus von Liebig
Justus von Liebig
Justus von Liebig was a German chemist who made major contributions to agricultural and biological chemistry, and worked on the organization of organic chemistry. As a professor, he devised the modern laboratory-oriented teaching method, and for such innovations, he is regarded as one of the...
the previous year), its properties were quite different. This finding challenged the prevailing chemical understanding of the time, which held that chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s could be different only when they had different elemental compositions. After additional discoveries of the same sort were made, such as Woehler's 1828 discovery that urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
had the same atomic composition as the chemically distinct ammonium cyanate, Jöns Jakob Berzelius
Jöns Jakob Berzelius
Jöns Jacob Berzelius was a Swedish chemist. He worked out the modern technique of chemical formula notation, and is together with John Dalton, Antoine Lavoisier, and Robert Boyle considered a father of modern chemistry...
introduced the term isomerism in 1830 to describe the phenomenon.
In 1848, Louis Pasteur
Louis Pasteur
Louis Pasteur was a French chemist and microbiologist born in Dole. He is remembered for his remarkable breakthroughs in the causes and preventions of diseases. His discoveries reduced mortality from puerperal fever, and he created the first vaccine for rabies and anthrax. His experiments...
separated tiny crystals of tartaric acid
Tartaric acid
Tartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to...
into their two mirror-image
Mirror image
A mirror image is a reflected duplication of an object that appears identical but reversed. As an optical effect it results from reflection off of substances such as a mirror or water. It is also a concept in geometry and can be used as a conceptualization process for 3-D structures...
forms. The individual molecules of each were the left and right optical stereoisomers, solutions of which rotate the plane of polarized light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
to the same degree but in opposite directions.
Other types of isomerism
Other types of isomerism exist outside this scope. Topological isomers called topoisomerTopoisomer
Topoisomers or topological isomers are molecules with the same chemical formula and stereochemical bond connectivities but different topologies. Examples of molecules for which there exist topoisomers include DNA, which can form knots, and catenanes...
s are generally large molecules that wind about and form different shaped knots or loops. Molecules with topoisomers include catenane
Catenane
A catenane is a mechanically-interlocked molecular architecture consisting of two or more interlocked macrocycles. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. Catenane is derived from the Latin catena meaning "chain"...
s and DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
. Topoisomerase
Topoisomerase
Topoisomerases are enzymes that regulate the overwinding or underwinding of DNA. The winding problem of DNA arises due to the intertwined nature of its double helical structure. For example, during DNA replication, DNA becomes overwound ahead of a replication fork...
enzymes can knot DNA and thus change its topology. There are also isotopomers
Isotopomers
Isotopomers or isotopic isomers are isomers with isotopic atoms, having the same number of each isotopic atom but differing in their positions. It can be either constitutional isomers or stereoisomers....
or isotopic
Isotopic
The word isotopic has a number of different meanings, including:* In the physical sciences, to do with chemical isotopes;* In mathematics, to do with a relation called isotopy.* In geometry, isotopic refers to facet-transitivity....
isomers that have the same numbers of each type of isotopic substitution but in chemically different positions. In nuclear physics
Nuclear physics
Nuclear physics is the field of physics that studies the building blocks and interactions of atomic nuclei. The most commonly known applications of nuclear physics are nuclear power generation and nuclear weapons technology, but the research has provided application in many fields, including those...
, nuclear isomer
Nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
s are excited states of atomic nuclei. Spin isomers
Spin isomers of hydrogen
Molecular hydrogen occurs in two isomeric forms, one with its two proton spins aligned parallel , the other with its two proton spins aligned antiparallel...
have differing distributions of spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
among their constituent atoms.
See also
- Nuclear isomerNuclear isomerA nuclear isomer is a metastable state of an atomic nucleus caused by the excitation of one or more of its nucleons . "Metastable" refers to the fact that these excited states have half-lives more than 100 to 1000 times the half-lives of the other possible excited nuclear states...
- Chirality (chemistry)Chirality (chemistry)A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
- Structural isomerismStructural isomerismStructural isomerism, or constitutional isomerism , is a form of isomerism in which molecules with the same molecular formula have bonded together in different orders, as opposed to stereoisomerism. There are multiple synonyms for constitutional isomers.Three categories of constitutional isomers...
- Cis-trans isomerism
- Cyclohexane conformationCyclohexane conformationA cyclohexane conformation is any of several three-dimensional shapes that a cyclohexane molecule can assume while maintaining the integrity of its chemical bonds....
- ElectromerismElectromerismElectromerism is a type of isomerism between a pair of molecules differing in the way electrons are distributed among the atoms and the connecting chemical bonds...