
Diastereomer
Encyclopedia
| Diastereomers | |
|---|---|
 |
 |
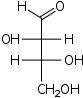
| D-Threose Threose Threose is a four-carbon monosaccharide or carbohydrate with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides... |
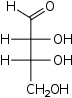
D-Erythrose Erythrose Erythrose is a tetrose carbohydrate with chemical formula C4H8O4. It has one aldehyde group and so is part of the aldose family. The natural isomer is D-erythrose.... |
- Erythro redirects here. For the fictional planet, see Erythro (Asimov).
Diastereomers (sometimes called diastereoisomers) are stereoisomers that are not enantiomer
Enantiomer
In chemistry, an enantiomer is one of two stereoisomers that are mirror images of each other that are non-superposable , much as one's left and right hands are the same except for opposite orientation. It can be clearly understood if you try to place your hands one over the other without...
s.
Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenter
Stereocenter
A stereocenter or stereogenic center is an atom, bearing groups such that an interchanging of any two groups leads to a stereoisomer.A chirality center is a stereocenter consisting of an atom holding a set of ligands in a spatial arrangement which is not superposable on its mirror image...
s and are not mirror images of each other.
When two diastereoisomers differ from each other at only one stereocenter they are epimer
Epimer
In chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
s. Each stereocenter gives rise to two different configurations and thus increases the number of stereoisomers by a factor of two.
Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another.
Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image.
Diastereomers have different physical properties and different reactivity, unlike enantiomers.
Cis-trans isomerism and conformational isomerism
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
are also forms of diastereomerism.
Diastereoselectivity is the preference for the formation of one or more than one diastereomer over the other in an organic reaction
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
.
Erythro / threo
Two common prefixes used to distinguish diastereomers are threo and erythro (which correspond to the more intuitive anti and syn labels, respectively). When drawn in the Fischer projectionFischer projection
The Fischer projection, devised by Hermann Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and...
the erythro isomer has two identical substituents on the same side and the threo isomer has them on opposite sides. When drawn as a zig-zag chain, the erythro isomer has two identical substituents on different sides of the plane (anti). The names are derived from the diastereomeric aldoses erythrose
Erythrose
Erythrose is a tetrose carbohydrate with chemical formula C4H8O4. It has one aldehyde group and so is part of the aldose family. The natural isomer is D-erythrose....
(a syrup) and threose
Threose
Threose is a four-carbon monosaccharide or carbohydrate with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides...
(melting point 126 °C).
Another threo compound is threonine
Threonine
Threonine is an α-amino acid with the chemical formula HO2CCHCHCH3. Its codons are ACU, ACA, ACC, and ACG. This essential amino acid is classified as polar...
, one of the essential amino acids. The erythro diastereomer is called allo-threonine.
Multiple stereocenters
If a molecule contains two asymmetric carbons, there are up to 4 possible configurations, and they cannot all be non-superimposable mirror images of each other. The possibilities continue to multiply as there are more asymmetric centers in a molecule. In general, the number of configurational isomers of a molecule can be determined by calculating 2n, where n = the number of chiralChirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
centers in the molecule. This holds true except in cases where the molecule has meso forms.
Example
Tartaric acidTartaric acid
Tartaric acid is a white crystalline diprotic organic acid. It occurs naturally in many plants, particularly grapes, bananas, and tamarinds; is commonly combined with baking soda to function as a leavening agent in recipes, and is one of the main acids found in wine. It is added to other foods to...
contains two asymmetric centers, but two of the "isomers" are equivalent and together are called a meso compound
Meso compound
A meso compound or meso isomer is a non-optically active member of a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters it is not chiral. A meso compound is superimposable on its mirror image, and it does not produce a ""...
. This configuration is not optically active, while the remaining two isomers are D- and L- mirror images, i.e., enantiomers. The meso form is a diastereomer of the other forms.
 |
 |
 |
(natural) tartaric acid L-(+)-tartaric acid dextrotartaric acid |
D-(-)-tartaric acid levotartaric acid |
mesotartaric acid |
(1:1) DL-tartaric acid "racemic acid" |
||
The families of 4, 5 and 6 carbon carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s contain many diastereomers because of the large numbers of asymmetric centres in these molecules.
Applications
As stated, two enantiomers will have identical chemical properties, while diastereomers will not. This knowledge is harnessed in chiral synthesisChiral synthesis
Enantioselective synthesis, also called chiral synthesis, asymmetric synthesis or stereoselective synthesis, is organic synthesis that introduces one or more new and desired elements of chirality...
to separate a mixture of enantiomers. This is the principle behind chiral resolution
Chiral resolution
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs...
. After preparing the diastereomers, they are separated by chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
or recrystallization
Recrystallization (chemistry)
-Chemistry:In chemistry, recrystallization is a procedure for purifying compounds. The most typical situation is that a desired "compound A" is contaminated by a small amount of "impurity B". There are various methods of purification that may be attempted , which includes recrystallization...
.
Therefore, for two diastereomers, two peaks are observed when analysed using NMR
NMR
NMR may refer to:Applications of Nuclear Magnetic Resonance:* Nuclear magnetic resonance* NMR spectroscopy* Solid-state nuclear magnetic resonance* Protein nuclear magnetic resonance spectroscopy* Proton NMR* Carbon-13 NMR...
.

