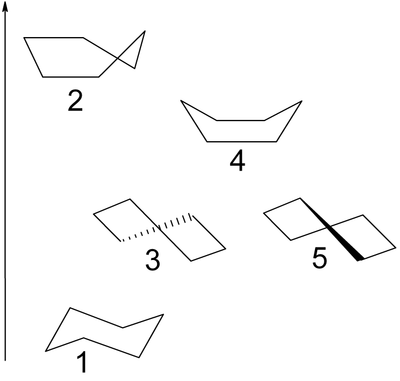
Cyclohexane conformation
Encyclopedia
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
can assume while maintaining the integrity of its chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s.
The internal angle
Internal angle
In geometry, an interior angle is an angle formed by two sides of a polygon that share an endpoint. For a simple, convex or concave polygon, this angle will be an angle on the 'inner side' of the polygon...
s of a flat regular
Regular polygon
A regular polygon is a polygon that is equiangular and equilateral . Regular polygons may be convex or star.-General properties:...
hexagon are 120 degrees, while the preferred angle between successive bonds
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
in a carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
chain is about 109 degrees. Therefore the cyclohexane ring tends to assume certain non-planar (warped) conformations, which have all angles closer to 109 degrees and therefore a lower strain energy
Strain energy
In a molecule, strain energy is released when the constituent atoms are allowed to rearrange themselves in a chemical reaction or a change of chemical conformation in a way that:* angle strain,* torsional strain,* ring strain and/or steric strain,...
than the flat hexagonal shape. The most important shapes are called chair, half-chair, boat, and twist-boat. The molecule can easily switch between these conformations, and only two of them — chair and twist-boat — can be isolated in pure form.
Cyclohexane conformations have been extensively studied in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
because they are the classical example of conformational isomerism
Conformational isomerism
In chemistry, conformational isomerism is a form of stereoisomerism in which the isomers can be interconverted exclusively by rotations about formally single bonds...
and have noticeable influence on the physical and chemical properties of cyclohexane.
Historical background
In 1890, Hermann Sachse, a 28-year-old assistant in Berlin, published instructions for folding a piece of paper to represent two forms of cyclohexane he called symmetrical and unsymmetrical (what we would now call chair and boat). He clearly understood that these forms had two positions for the hydrogens (again, to use modern terminology, axial and equatorial), that two chairs would probably interconvert, and even how certain substituents might favor one of the chair forms. Because he expressed all this in mathematical language, few chemists of the time understood his arguments. He had several attempts at publishing these ideas, but none succeeded in capturing the imagination of chemists. His death in 1893 at the age of 31 meant his ideas sank into obscurity. It was only in 1918 when Ernst Mohr, using the then very new technique of x-ray crystallography, was able to determine the molecular structure of diamondDiamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
, that it became recognised that Sachse's chair was the pivotal motif.
Derek Barton and Odd Hassel
Odd Hassel
Odd Hassel was a Norwegian physical chemist and Nobel Laureate.-Biography:Born in Kristiania, his parents were Ernst Hassel, a gynaecologist, and Mathilde Klaveness. In 1915, he entered the University of Oslo where he studied mathematics, physics and chemistry, and graduated in 1920...
shared the 1969 Nobel Prize
Nobel Prize
The Nobel Prizes are annual international awards bestowed by Scandinavian committees in recognition of cultural and scientific advances. The will of the Swedish chemist Alfred Nobel, the inventor of dynamite, established the prizes in 1895...
for work on the conformations of cyclohexane and various other molecules.
General
The carbon-carbon bonds along the cyclohexane ring are sp³ hybrid orbitalsOrbital hybridisation
In chemistry, hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals suitable for the qualitative description of atomic bonding properties. Hybridised orbitals are very useful in the explanation of the shape of molecular orbitals for molecules. It is an integral part...
, which have tetrahedral
Tetrahedron
In geometry, a tetrahedron is a polyhedron composed of four triangular faces, three of which meet at each vertex. A regular tetrahedron is one in which the four triangles are regular, or "equilateral", and is one of the Platonic solids...
symmetry. Therefore, the angles between bonds of a tetravalent carbon atom have a preferred value θ ≈ 109.5°. The bonds also have a fairly fixed bond length
Molecular geometry
Molecular geometry or molecular structure is the three-dimensional arrangement of the atoms that constitute a molecule. It determines several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism, and biological activity.- Molecular geometry determination...
λ. On the other hand, adjacent carbon atoms are free to rotate about the axis of the bond. Therefore, a ring that is warped so that the bond lengths and angles are close to those ideal values will have less strain energy than a flat ring with 120° angles.
For each particular conformation of the carbon ring, the directions of the 12 carbon-hydrogen bonds (and therefore the positions of the hydrogen atoms) are fixed.
There are exactly eight warped polygon
Polygon
In geometry a polygon is a flat shape consisting of straight lines that are joined to form a closed chain orcircuit.A polygon is traditionally a plane figure that is bounded by a closed path, composed of a finite sequence of straight line segments...
s with six distinguished corners that have all internal angles equal to θ and all sides equal to λ. They comprise two ideal chair conformations, where the carbons alternately lie above and below the mean ring plane; and six ideal boat conformations, where two opposite carbons lie above the mean plane, and the other four lie below it. In theory, a molecule with any of those ring conformations would be free of angle strain
Angle strain
Angle strain, also called Baeyer strain in cyclic molecules, is the resistance associated with bond angle compression or bond angle expansion. It occurs when bond angles deviate from the ideal bond angles to achieve maximum bond strength in a specific chemical conformation...
. However, due to interactions between the hydrogen atoms, the angles and bond lengths of the actual chair forms are slightly different from the nominal values. For the same reasons, the actual boat forms have slightly higher energy than the chair forms. Indeed, the boat forms are unstable, and deform spontaneously to twist-boat conformations that are local minima of the total energy, and therefore stable.
Each of the stable ring conformations can be transformed into any other without breaking the ring. However, such transformations must go through other states with stressed rings. In particular, they must go through unstable states where four successive carbon atoms lie on the same plane. These shapes are called half-chair conformations.
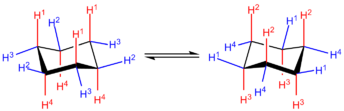
Chair conformation
The two chair conformations have the lowest total energy, and are therefore the most stable. In the basic chair conformation, the carbons C1 through C6 alternate between two parallel planes, one with C1, C3 and C5, the other with C2, C4, and C6. The molecule has a symmetry axis perpendicular to these two planes, and is congruent to itself after a rotation of 120° about that axis. The two chair conformations have the same shape; one is congruent to the other after 60° rotation about that axis, or after being mirrored across the mean plane. The perpendicular projectionof the ring onto its mean plane is a regular hexagon. All C-C bonds are tilted relative to the mean plane, but opposite bonds (such as C1-C2 and C4-C5) are parallel to each other.
As a consequence of the ring warping, six of the 12 carbon-hydrogen bonds end up almost perpendicular to the mean plane and almost parallel to the symmetry axis, with alternating directions, and are said to be axial. The other six C-H bonds lie almost parallel to the mean plane, and are said to be equatorial.
Staggered
In organic chemistry, a staggered conformation is a chemical conformation of an ethane-like moiety abcX-Ydef in which the substituents a,b,and c are at the maximum distance from d,e,and f...
so that there is little torsional strain. This geometry is often preserved when the hydrogen atoms are replaced by halogen
Halogen
The halogens or halogen elements are a series of nonmetal elements from Group 17 IUPAC Style of the periodic table, comprising fluorine , chlorine , bromine , iodine , and astatine...
s or other simple group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s.
The conversion from one chair shape to the other is called ring flip
Ring flip
Ring flipping is a phenomenon involving the interconversion about single bonds of cyclic conformers having equivalent ring shapes but not necessarily equivalent spatial positions of substituent atoms.The six-membered ring of the alkane, cyclohexane, has the 'chair' as its preferred conformation...
ping or chair-flipping. Carbon-hydrogen bonds that are axial in one configuration become equatorial in the other, and vice-versa; but their "up" or "down" character remains the same.
In cyclohexane, the two chair conformations have the same energy. At 25°C, 99.99% of all molecules in a cyclohexane solution will be in a chair conformation.
In cyclohexane derivatives, the two chair conformations may have different energies, depending upon the identity and location of the substituents. For example, in methylcyclohexane
Methylcyclohexane
Methylcyclohexane is a colourless liquid with a faint benzene-like odour. Its molecular formula is C7H14. Methylcyclohexane is used in organic synthesis and as a solvent for cellulose ethers. It is a component of jet fuel and is also a component of correction fluids.-Structure:Monosubstituted...
the lowest energy conformation is a chair one where the methyl group is in equatorial position. This configuration reduces interaction between the methyl group (on carbon number 1) and the hydrogens at carbons 3 and 5; more importantly, it avoids two gauche butane interactions (of the C1-CH3 bond with the C2-C3 and C5-C6 ring bonds). Similarly, cis-1,3-dimethylcyclohexane usually has both methyls in the equatorial position so as to avoid interaction between them. In six-membered heterocyles such as pyran
Pyran
In chemistry, a pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the location of the double bonds...
, a substituent next to an heteroatom may prefer the axial position due to the anomeric effect
Anomeric effect
In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the less hindered equatorial orientation that would...
.
The preference of a substituent towards the equatorial conformation is measured in terms of its A value
A value
thumb|400px|right| The A-value for a [[methyl]] group is 1.74 as derived from the [[chemical equilibrium]] above. This means it costs 1.74 [[kcal/mol]] of energy to have a methyl group in the axial position compared to the equatorial position....
, which is the Gibbs free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
difference between the two chair conformations, with the substituent in equatorial or in axial position. A positive A value indicates preference towards the equatorial position. The magnitude of the A values ranges from nearly zero for very small substituents such as deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
, to about 5 kcal/mol for very bulky substituents such as the tert-butyl group.
Boat conformation
In the basic boat conformation (C2v symmetryMolecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
), carbons C2, C3, C5 and C6 are coplanar, while C1 and C4 are displaced away from that plane in the same direction. Bonds C2-C3 and C5-C6 are therefore parallel. In this form, the molecule has two perpendicular planes of symmetry as well as a C2 axis
Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
. The boat conformations have higher energy than the chair conformations. The interaction between the two flagpole hydrogens, in particular, generates steric strain. There is also torsional strain involving the C2-C3 and C5-C6 bonds, which are eclipsed
Eclipsed
In chemistry an eclipsed conformation is a conformation in which two substituents X and Y on adjacent atoms A, B are in closest proximity, implying that the torsion angle X-A-B-Y is 0°. Such a conformation exists in any open chain single chemical bond connecting two sp3 hybridised atoms, and is...
. Because of this strain, the boat configuration is unstable (not a local minimum of the energy function).
Twist-boat conformation
The twist-boat conformation, sometimes called twist (D2 symmetryMolecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
) can be derived from the boat conformation by applying a slight twist to the molecule about the axes connecting the two unique carbons. The result is a structure that has three C2 axes and no plane of symmetry.
The concentration of the twist-boat conformation at room temperature is very low (less than 0.1%) but at 1073 Kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
it can reach 30%. Rapid cooling from 1073 K to 40 K will freeze in a large concentration of twist-boat conformation, which will then slowly convert to the chair conformation upon heating .
Half-chair conformation
The half-chair conformation is a transition state with C2 symmetryMolecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
generally considered to be on the pathway between chair and twist-boat. It involves rotating one of the dihedrals to zero such that four adjacent atoms are coplanar and the other two atoms are out of plane (one above and one below).
Interconversions between conformations
At room temperature there is a rapid equilibriumChemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
between the two chair conformations of cyclohexane. The interconversion of these two conformations has been much debated and still lacks consensus. What is known is that the twist-boat and chair are both energy minima—the twist-boat being a local minimum
Maxima and minima
In mathematics, the maximum and minimum of a function, known collectively as extrema , are the largest and smallest value that the function takes at a point either within a given neighborhood or on the function domain in its entirety .More generally, the...
; the chair being a global minimum
Maxima and minima
In mathematics, the maximum and minimum of a function, known collectively as extrema , are the largest and smallest value that the function takes at a point either within a given neighborhood or on the function domain in its entirety .More generally, the...
(ground state
Ground state
The ground state of a quantum mechanical system is its lowest-energy state; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state...
).
The half-chair state (2, below) is the transition state
Transition state
The transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
in the interconversion between the chair and twist-boat conformations. Due to the D2 symmetry
Molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
of the twist-boat, there are two energy-equivalent pathways that it can take to two different half-chair conformations, leading to the two different chair conformations of cyclochexane. Thus, at a minimum, the interconversion between the two chair conformations involves the following sequence: chair - half-chair - twist-boat - half-chair' - chair'.
The conformations involve following order of stability: chair form > twist boat form > boat form > half-chair form.
The boat conformation (4, below) is also a transition state, allowing the interconversion between two different twist-boat conformations. While the boat conformation is not necessary for interconversion between the two chair conformations of cyclohexane, it is often included in the reaction coordinate diagram
Reaction coordinate
In chemistry, a reaction coordinate is an abstract one-dimensional coordinate which represents progress along a reaction pathway. It is usually a geometric parameter that changes during the conversion of one or more molecular entities....
used to describe this interconversion because its energy is considerably lower than that of the half-chair, so any molecule with enough energy to go from twist-boat to chair also has enough energy to go from twist-boat to boat. Thus, there are multiple pathways by which a molecule of cyclohexane in the twist-boat conformation can achieve the chair conformation again.
Forced conformations
[6.6]Chiralane is a point group T molecule wholly composed of identical fused twist-boat cyclohexanes. TwistaneTwistane
Twistane is an organic compound with the formula C10H16. It is a cycloalkane and an isomer of the simplest diamondoid, adamantane, and like adamantane, is very volatile...
is another compound with a forced twist-boat conformation.
Cyclohexane derivatives
Substituents found on cyclohexane adopt cis and trans formations and cannot be easily switched by simple single sigma bond rotation as with linear molecules. Cis formation means that both substituents are found on the upper side of the 2 substituent placements on the carbon, while trans would mean that they were on opposing sides. Despite the fact that carbons on cyclohexane are linked by a single bond, the ring remains rigid, in that switching from cis to trans would require breaking the ring. The nomenclature for cis is dubbed (Z) while the name for trans is (E) to be placed in front of the IUPAC name.For di-substituted cyclohexane rings (i.e. two groups on the ring), the relative orientation of the two substituents affect the energy of the possible conformations. For 1,2- and 1,4-di-substituted cyclohexane, a cis configuration leads to one axial and one equatorial group. This configuration can undergo chair flipping. For 1,2- and 1,4-di-substituted cyclohexane, a trans configuration leads to either both groups axial or both equatorial. In this case, the diaxial conformation is effectively prevented by its high steric strain (four gauche interactions more than the diequatorial). For 1,3-di-substituted cyclohexanes, the cis form is diequatorial and the flipped conformation suffers additional steric interaction between the two axial groups. Trans-1,3-di-substituted cyclohexanes are like cis-1,2- and cis-1,4- and can flip between the two equivalent axial/equatorial forms.
Derivatives of cyclohexane do exist that have a more stable twist-boat conformation. An example is 1,2,4,5-tetrathiane, an organosulfur compound with 4 methylene
Methylene
Methylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
groups replaced by a sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
group thus removing unfavorable 1,3-diaxial interactions. In the tetramethyl analogue 3,3,6,6-tetramethyl-1,2,4,5-tetrathiane the twist-boat conformation actually dominates. Also in cyclohexane-1,4-dione with the steric 1,4-hydrogen interaction removed, the actual stable conformation is the twist-boat.
Cis-1,4-di-tert-butylcyclohexane has an axial tert-butyl group in the chair conformation and conversion to the twist-boat conformation places both groups in more favorable equatorial positions. As a result the twist-boat conformation is more stable by 0.47 kcal/mol (1.96 kJ/mol) at 125 K as measured by NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
.
Effect of polar substituent :
- cis-cyclohexane-1,3-diol prefers diaxial conformation "formation of intrahydrogen bond".
- 2,5-di-tert-butyl-1,4-cyclohexanediol present in boat or twist-boat form "also intra-H-bond"
- 2-bromocyclohexanone prefers a-Br "min.dipolar repulsion"
- 2-bromo-4,4-dimethylcyclohexanone prefers e-Br "1,3 diaxial interaction(-ve in e-Br)more than
dipolar repulsion :
- trans-1,2-dibromocyclohexane present in axial form in non-polar solvents "dipoles cancel"
while present in equtorial form in polar solvents "dipoles reinforce".
External links
- Java applets of all conformations from the University of Nijmegen
- Ring Conformations & Sterioisomers Detailed description of ring conformations and sterioisomers from Michigan State UniversityMichigan State UniversityMichigan State University is a public research university in East Lansing, Michigan, USA. Founded in 1855, it was the pioneer land-grant institution and served as a model for future land-grant colleges in the United States under the 1862 Morrill Act.MSU pioneered the studies of packaging,...