
Thalidomide
Encyclopedia
Thalidomide was introduced as a sedative
drug in the late 1950s that was typically used to cure morning sickness
. In 1961, it was withdrawn due to teratogenicity
and neuropathy
. There is now a growing clinical interest in thalidomide, and it is introduced as an immunomodulatory agent used primarily in combination with dexamethasone
to treat multiple myeloma
. The drug is a potent teratogen in zebrafish, chicken
s, rabbit
s and primate
s, including humans; severe birth defects have been reported at an exceptional level in individuals whose mother took the drug during pregnancy.
Thalidomide was sold in a number of countries across the world from 1957 until 1961, when it was withdrawn from the market after being found to be a cause of birth defects in what has been called "one of the biggest medical tragedies of modern times". It is not known exactly how many worldwide victims of the drug there have been, although estimates range from 10,000 to 20,000.
Thalidomide has since been found to be a viable treatment for a number of medical conditions. It is being prescribed again in a number of countries, although its use, including its testing in the developing world, remains controversial. The thalidomide tragedy led to much stricter testing being required for drugs and pesticides before they can be licensed.
in Stolberg (Rhineland) near Aachen
. A report published by Martin W. Johnson, director of the Thalidomide Trust in the United Kingdom, mentioned evidence found by Argentinian author Carlos De Napoli that suggested the drug had been first developed as a possible antidote to nerve toxins, such as Sarin
, by Otto Ambros
, a Nazi scientist who joined Grünenthal after the war. Correspondence between various drug companies -- French firm Rhône-Poulenc
, which was under Nazi control during the war years, Astra AB
, which held the Swedish licence to distribute thalidomide, and IG Farben
, the German pharmaceutical firm -- seem to confirm the existence of the product years before Grünenthal secured a patent in 1954. Furthermore, a relation between testing thalidomide and the Nazi death camps has been suggested.
Grünenthal has responded to these claims by stating, "To our knowledge there was no collaboration between Grünenthal and Rhône-Poulenc for the development of Contergan/thalidomide. Three Grünenthal employees discovered thalidomide and Grünenthal is the sole inventor on the patent." According to Grünenthal, Dr. Heinrich Mückter was one of those responsible for inventing thalidomide. Other sources mark Dr. Mückter as a fledgling pharmacologist who carried out wartime experiments on Polish prisoners to find a cure for typhus, causing the death of hundreds in the process.
De Napoli suggested elsewhere that thalidomide may have been first synthesised
by British scientists at the University of Nottingham
in 1949.
Thalidomide, launched by Grünenthal on 1 October 1957, was found to act as an effective tranquilizer and painkiller, and was proclaimed a "wonder drug" for insomnia, coughs, colds and headaches. It was also found to be an effective antiemetic
that has an inhibitory effect on morning sickness
, so thousands of pregnant women took the drug to relieve their symptoms. At the time of the drug's development, scientists did not believe any drug taken by a pregnant woman could pass across the placental barrier and harm the developing fetus
. The Food and Drug Administration of the United States never licensed thalidomide for general use; according to Time Magazine, "In the half dozen reported U.S. cases of birth malformations due to thalidomide, the drug was obtained from abroad." However, samples had been distributed to a number of physicians as part of a clinical trial, in which 20,000 patients in the U.S. received thalidomide.
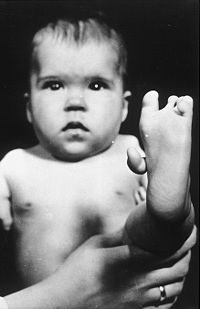 In the late 1950s and early 1960s, more than 10,000 children in 46 countries were born with deformities, such as phocomelia
In the late 1950s and early 1960s, more than 10,000 children in 46 countries were born with deformities, such as phocomelia
, as a consequence of thalidomide use. The Australian obstetrician William McBride and the German pediatrician Widukind Lenz
suspected a link between birth defects and the drug, a theory Lenz proved in 1961. McBride was later awarded a number of honours, including a medal and prize money by the prestigious L'Institut de la Vie in Paris.
In the United Kingdom, the drug was licensed in 1958. Of the approximately 2,000 babies born with defects, 466 survived. The drug was withdrawn in 1961. In 1968, after a long campaign by The Sunday Times
newspaper, a compensation settlement for the UK victims was reached with Distillers Company (now part of Diageo
). This compensation, which is distributed by the Thalidomide Trust in the UK, was substantially increased by Diageo in 2005. The UK Government gave survivors a grant of £20 million, to be distributed through the Thalidomide Trust, in December 2009. In Germany approximately 2,500 thalidomide babies were born.
 In the United States, pharmacologist and M.D. Frances Oldham Kelsey
In the United States, pharmacologist and M.D. Frances Oldham Kelsey
refused Food and Drug Administration (FDA) approval for an application from the Richardson-Merrell
company to market thalidomide, saying further studies were needed, which reduced the impact of thalidomide in United States patients. Although thalidomide was never approved for sale in the United States, millions of tablets had been distributed to physicians during a clinical testing program. It was impossible to know how many pregnant women had been given the drug to help alleviate morning sickness or as a sedative.
Canada was the last country to stop the sales of the drug, in early 1962.
In 1962, the United States Congress
enacted laws requiring tests for safety during pregnancy before a drug can receive approval for sale in the U.S. Other countries enacted similar legislation, and thalidomide was not prescribed or sold for decades.
For correctly denying the application despite the pressure from Richardson-Merrell, Kelsey eventually received the President's Award for Distinguished Federal Civilian Service at a 1962 ceremony with President John F. Kennedy
. In September 2010, as noted in an article titled "The Public's Quiet Savior From Harmful Medicine", the FDA honored Dr. Kelsey with the first Kelsey award. The award, given annually to a FDA staff member, came 50 years after Dr. Kelsey, then a new medical officer at the agency, first reviewed the application from the William S. Merrell Company of Cincinnati.
, Professor at the Hebrew University of Jerusalem
at Hadassah University Hospital and the chief staff and manager of Hansen Leper Hospital in Jerusalem, administered thalidomide to a critically ill patient with erythema nodosum leprosum (ENL)
, a painful complication of leprosy
, in an attempt to relieve his pain in spite of the ban. The patient slept for hours, and was able to get out of bed without aid upon awakening. The result was followed by more favorable experiences and then by a clinical trial. Sheskin found that patients with ENL, a painful skin condition, experienced pain relief when taking thalidomide.
Further work conducted in 1991 by Dr. Gilla Kaplan at Rockefeller University
in New York City showed thalidomide worked in leprosy by inhibiting tumor necrosis factor alpha. Kaplan believed thalidomide could be an effective treatment for AIDS. He partnered with Celgene to further develop the potential for thalidomide in AIDS and tuberculosis. However, clinical trials for AIDS proved disappointing.
In 1994, Dr. Robert D'Amato at Harvard Medical School discovered thalidomide was a potent inhibitor of new blood vessel growth (angiogenesis). Numerous cancer clinical trials for thalidomide began based upon this finding. In 1997, Dr. Bart Barlogie reported thalidomide's initial effectiveness against multiple myeloma
, and thalidomide was later approved in the United States by the FDA for use in this malignancy. The FDA has also approved the drug's use in the treatment of ENL. Studies are underway to determine the drug's effects on arachnoiditis
and several types of cancer
s. However, physicians and patients alike must go through a special process, known as STEPS, to prescribe and receive thalidomide, to ensure no more children are born with birth defects traceable to the medication. Celgene has also developed analogues to thalidomide, such as lenalidomide
, that are substantially more powerful and have fewer side effects — except for greater myelosuppression. Lenalidomide is now more commonly used than thalidomide for myeloma.
More recently, the World Health Organisation (WHO) has stated:
. Because of thalidomide's potential for causing birth defects, the drug may be distributed only under tightly controlled conditions. The FDA required that Celgene Corporation, which planned to market thalidomide under the brand name Thalomid, establish a system for thalidomide education and prescribing safety (STEPS) oversight program. The conditions required under the program include limiting prescription and dispensing rights only to authorized prescribers and pharmacies, keeping a registry of all patients prescribed thalidomide, providing extensive patient education about the risks associated with the drug, and providing periodic pregnancy tests for women who take the drug.
On May 26, 2006, the U.S. Food and Drug Administration granted accelerated approval for thalidomide (Thalomid, Celgene Corporation) in combination with dexamethasone for the treatment of newly diagnosed multiple myeloma
(MM) patients. The FDA approval came seven years after the first reports of efficacy in the medical literature and Celgene took advantage of "off-label" marketing opportunities to promote the drug in advance of its FDA approval for the myeloma indication. Thalomid, as the drug is commercially known, sold over $300 million per year, while approved only for leprosy.
in the world, and thalidomide has been used by Brazilian physicians as the drug of choice for the treatment of severe ENL since 1965. A study published in 1996 reported 33 people born in Brazil after 1965 with thalidomide embryopathy. Since 1994, the production, dispensing, and prescription of thalidomide have been strictly controlled, but cases of thalidomide embryopathy continue.
and tuberculosis
cause the level of tumor necrosis factor-alpha (TNFα) to rise. TNFα is a chemical mediator in the body, and may enhance the wasting process in cancer patients, as well. Thalidomide may reduce the levels of TNFα, and it is possible that the drug's effect on ENL is caused by this mechanism.
Thalidomide also has potent anti-inflammatory effects that may help ENL patients. In July 1998, the FDA approved the application of Celgene
to distribute thalidomide under the brand name Thalomid for treatment of ENL. Pharmion Corporation, who licensed the rights to market thalidomide in Europe, Australia, and various other territories from Celgene, received approval for its use against multiple myeloma
in Australia
and New Zealand
in 2003. Thalomid, in conjunction with dexamethasone
, is now standard therapy for multiple myeloma.
Thalidomide is also prescribed for its anti-inflammatory effects in actinic prurigo
, an autoimmune skin disease.
Thalidomide has been used in chronic bullous dermatosis of childhood (CBDC) with encouraging results. Peripheral neuritis may be a limiting factor for long term use of thalidomide.
Thalidomide also inhibits the growth of new blood vessels (angiogenesis
), which may be useful in treating macular degeneration
and other diseases. This effect helps AIDS
patients with Kaposi's sarcoma
, although there are better and cheaper drugs to treat the condition. Thalidomide may be able to fight painful, debilitating aphthous lesions in the mouth and esophagus
of AIDS patients that prevent them from eating. The FDA formed a Thalidomide Working Group in 1994 to provide consistency between its divisions, with particular emphasis on safety monitoring. The agency also imposed severe restrictions on the distribution of Thalomid through the STEPS program.
Thalidomide is also being investigated for treating symptoms of prostate cancer
, glioblastoma, lymphoma
, arachnoiditis
, Behçet's disease
, and Crohn's disease
. In a small trial, Australian researchers found thalidomide caused a doubling of the number of T cell
s in patients, allowing the patients' own immune system
to attack cancer cells.
Studies carried out in animal models have suggested the use of combined therapy with thalidomide and glucantime could have a therapeutic benefit in the treatment of visceral leshmaniasis.
A study published in April 2010 discussed the ability of thalidomide to induce vessel maturation, which may be useful as a therapeutic strategy for the treatment of vascular malformations. The research was conducted in an experimental model of the genetic disease hereditary hemorrhagic telangiectasia
.
in 1996 due to its antiangiogenic activity. The New England Journal of Medicine published the full study in 1999. Since then, many studies have shown that thalidomide, in combination with dexamethasone
, has increased the survival of multiple myeloma patients. The combination of thalidomide and dexamethasone, often in combination with melphalan
, is now one of the most common regimens for patients with newly diagnosed multiple myeloma, with an improved response rate of up to 60-70%. Thalidomide may also cause side effects, such as polyneuropathy
, fatigue, skin rash, and venous thromboembolism (VTE), or blood clots, which could lead to stroke
or myocardial infarction
. Bennett et al. have conducted a systematic review of VTE associated with thalidomide in multiple myeloma patients. They have found that when thalidomide was administered without prophylaxis, VTE rates reached as high as 26%. Owing to the high rates of VTE associated with thalidomide in combination with dexamethasone or doxorubicin
, a black box warning
was added in the US in 2006 to the package insert for thalidomide, indicating that patients with multiple myeloma who receive thalidomide-dexamethasone may benefit from concurrent thromboembolism prophylaxis or aspirin
. In addition, owing to these side effects, newer drugs, such as bortezomib
(marketed as Velcade) and a thalidomide derivative, lenalidomide
(marketed as Revlimid), have increased in popularity.
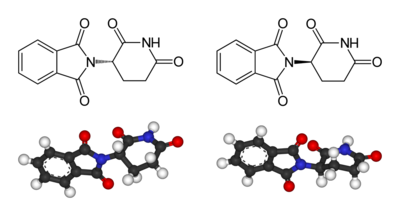 Thalidomide is racemic
Thalidomide is racemic
– it contains both left- and right-handed isomer
s in equal amounts. The (R) enantiomer is effective against morning sickness, but the (S) is teratogenic
. The enantiomers can interconvert (racemize) in vivo
– that is, if a human is given pure (R)-thalidomide or (S)-thalidomide, both isomers will later be found in the serum
– therefore, administering only one enantiomer will not prevent the teratogenic effect.
The mechanism of thalidomide's teratogenic action has led to over 2000 research papers and the proposal of 15 or 16 plausible mechanisms. A theoretical synthesis in 2000 suggested the following mechanism: thalidomide intercalates
(inserts itself) into DNA
in guanine
-cytosine
-rich regions. Owing to its glutarimide part, (S) thalidomide fits neatly into the major groove of DNA at purine sites. Such intercalation impacts upon the promoter regions of the genes controlling the development of limbs, ears, and eyes, such as IGF-I and FGF-2. These normally activate the production of the cell surface attachment integrin
αvβ3, with the resulting αvβ3 integrin dimer stimulating angiogenesis
in developing limb buds. This then promotes the outgrowth of the bud (IGF-I and FGF-2 are also both known to stimulate angiogenesis). Therefore, by inhibiting the chain of events, thalidomide causes the truncation of limb development. In 2009, this theory received strong support, with research showing "conclusively that loss of newly formed blood vessels is the primary cause of thalidomide teratogenesis, and developing limbs are particularly susceptible because of their relatively immature, highly angiogenic vessel network."
, which is important in limb formation. The inactivation leads to a teratogenic effect on fetal development
. This was confirmed when the scientists, using genetic techniques, reduced the production of cereblon
in developing chick and zebrafish embryos. These embryos had defects similar to those treated with thalidomide. While the mechanism that causes teratogenicity has been established, the mechanism for other therapeutic effects remains unclear.
by several mechanisms, as resulting mainly from experiments on myeloma cancer cell lines:
s. In 2005, Celgene received FDA approval for lenalidomide
(Revlimid) as the first commercially useful derivative. Revlimid is available only in a restricted distribution setting to avoid its use during pregnancy. Further studies are being conducted to find safer compounds with useful qualities. Another analog, pomalidomide
, is in the clinical trial phase. These thalidomide analogs can be used to treat different diseases, or used in a regimen to fight two conditions.
Sedative
A sedative or tranquilizer is a substance that induces sedation by reducing irritability or excitement....
drug in the late 1950s that was typically used to cure morning sickness
Morning sickness
Morning sickness, also called nausea gravidarum, nausea, vomiting of pregnancy , or pregnancy sickness is a condition that affects more than half of all pregnant women. Related to increased oestrogen levels, a similar form of nausea is also seen in some women who use hormonal contraception or...
. In 1961, it was withdrawn due to teratogenicity
Teratology
Teratology is the study of abnormalities of physiological development. It is often thought of as the study of human birth defects, but it is much broader than that, taking in other non-birth developmental stages, including puberty; and other non-human life forms, including plants.- Etymology :The...
and neuropathy
Peripheral neuropathy
Peripheral neuropathy is the term for damage to nerves of the peripheral nervous system, which may be caused either by diseases of or trauma to the nerve or the side-effects of systemic illness....
. There is now a growing clinical interest in thalidomide, and it is introduced as an immunomodulatory agent used primarily in combination with dexamethasone
Dexamethasone
Dexamethasone is a potent synthetic member of the glucocorticoid class of steroid drugs. It acts as an anti-inflammatory and immunosuppressant...
to treat multiple myeloma
Multiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
. The drug is a potent teratogen in zebrafish, chicken
Chicken
The chicken is a domesticated fowl, a subspecies of the Red Junglefowl. As one of the most common and widespread domestic animals, and with a population of more than 24 billion in 2003, there are more chickens in the world than any other species of bird...
s, rabbit
Rabbit
Rabbits are small mammals in the family Leporidae of the order Lagomorpha, found in several parts of the world...
s and primate
Primate
A primate is a mammal of the order Primates , which contains prosimians and simians. Primates arose from ancestors that lived in the trees of tropical forests; many primate characteristics represent adaptations to life in this challenging three-dimensional environment...
s, including humans; severe birth defects have been reported at an exceptional level in individuals whose mother took the drug during pregnancy.
Thalidomide was sold in a number of countries across the world from 1957 until 1961, when it was withdrawn from the market after being found to be a cause of birth defects in what has been called "one of the biggest medical tragedies of modern times". It is not known exactly how many worldwide victims of the drug there have been, although estimates range from 10,000 to 20,000.
Thalidomide has since been found to be a viable treatment for a number of medical conditions. It is being prescribed again in a number of countries, although its use, including its testing in the developing world, remains controversial. The thalidomide tragedy led to much stricter testing being required for drugs and pesticides before they can be licensed.
Development
Thalidomide was developed by German pharmaceutical company GrünenthalGrünenthal
Grünenthal GmbH is a German pharmaceutical company in Stolberg near Aachen, which holds the patent to Ultram , and its much stronger derivative Nucynta , both used as analgesics with Norepinephrine Reuptake Inhibition...
in Stolberg (Rhineland) near Aachen
Aachen
Aachen has historically been a spa town in North Rhine-Westphalia, Germany. Aachen was a favoured residence of Charlemagne, and the place of coronation of the Kings of Germany. Geographically, Aachen is the westernmost town of Germany, located along its borders with Belgium and the Netherlands, ...
. A report published by Martin W. Johnson, director of the Thalidomide Trust in the United Kingdom, mentioned evidence found by Argentinian author Carlos De Napoli that suggested the drug had been first developed as a possible antidote to nerve toxins, such as Sarin
Sarin
Sarin, or GB, is an organophosphorus compound with the formula [2CHO]CH3PF. It is a colorless, odorless liquid, which is used as a chemical weapon. It has been classified as a weapon of mass destruction in UN Resolution 687...
, by Otto Ambros
Otto Ambros
Otto Ambros was a German chemist, notably involved with the research of chemical nerve agents.-Early life:He was the son of a university professor. He went to school and passed his Abitur exam in Munich. In 1920 he went to the University of Munich to study chemistry and agricultural science...
, a Nazi scientist who joined Grünenthal after the war. Correspondence between various drug companies -- French firm Rhône-Poulenc
Rhône-Poulenc
-History of the company:The Company was founded in 1928 through the merger of Société des Usines Chimiques du Rhône from Lyon and Établissements Poulenc Frères from Paris founded by Étienne Poulenc, a 19th century Parisian apothecary and brought to prominence by his second and third sons Emile...
, which was under Nazi control during the war years, Astra AB
Astra AB
Astra AB was a former international pharmaceutical company headquartered in Södertälje, Sweden. Astra was formed in 1913 and merged with the British Zeneca Group in 1999 to form AstraZeneca. Product development was focused on therapeutics for gastrointestinal, cardiovascular and respiratory...
, which held the Swedish licence to distribute thalidomide, and IG Farben
IG Farben
I.G. Farbenindustrie AG was a German chemical industry conglomerate. Its name is taken from Interessen-Gemeinschaft Farbenindustrie AG . The company was formed in 1925 from a number of major companies that had been working together closely since World War I...
, the German pharmaceutical firm -- seem to confirm the existence of the product years before Grünenthal secured a patent in 1954. Furthermore, a relation between testing thalidomide and the Nazi death camps has been suggested.
Grünenthal has responded to these claims by stating, "To our knowledge there was no collaboration between Grünenthal and Rhône-Poulenc for the development of Contergan/thalidomide. Three Grünenthal employees discovered thalidomide and Grünenthal is the sole inventor on the patent." According to Grünenthal, Dr. Heinrich Mückter was one of those responsible for inventing thalidomide. Other sources mark Dr. Mückter as a fledgling pharmacologist who carried out wartime experiments on Polish prisoners to find a cure for typhus, causing the death of hundreds in the process.
De Napoli suggested elsewhere that thalidomide may have been first synthesised
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
by British scientists at the University of Nottingham
University of Nottingham
The University of Nottingham is a public research university based in Nottingham, United Kingdom, with further campuses in Ningbo, China and Kuala Lumpur, Malaysia...
in 1949.
Thalidomide, launched by Grünenthal on 1 October 1957, was found to act as an effective tranquilizer and painkiller, and was proclaimed a "wonder drug" for insomnia, coughs, colds and headaches. It was also found to be an effective antiemetic
Antiemetic
An antiemetic is a drug that is effective against vomiting and nausea. Antiemetics are typically used to treat motion sickness and the side effects of opioid analgesics, general anaesthetics, and chemotherapy directed against cancer....
that has an inhibitory effect on morning sickness
Morning sickness
Morning sickness, also called nausea gravidarum, nausea, vomiting of pregnancy , or pregnancy sickness is a condition that affects more than half of all pregnant women. Related to increased oestrogen levels, a similar form of nausea is also seen in some women who use hormonal contraception or...
, so thousands of pregnant women took the drug to relieve their symptoms. At the time of the drug's development, scientists did not believe any drug taken by a pregnant woman could pass across the placental barrier and harm the developing fetus
Fetus
A fetus is a developing mammal or other viviparous vertebrate after the embryonic stage and before birth.In humans, the fetal stage of prenatal development starts at the beginning of the 11th week in gestational age, which is the 9th week after fertilization.-Etymology and spelling variations:The...
. The Food and Drug Administration of the United States never licensed thalidomide for general use; according to Time Magazine, "In the half dozen reported U.S. cases of birth malformations due to thalidomide, the drug was obtained from abroad." However, samples had been distributed to a number of physicians as part of a clinical trial, in which 20,000 patients in the U.S. received thalidomide.
Birth defects

Phocomelia
Phocomelia is an extremely rare congenital disorder involving the limbs . Étienne Geoffroy Saint-Hilaire coined the term in 1836....
, as a consequence of thalidomide use. The Australian obstetrician William McBride and the German pediatrician Widukind Lenz
Widukind Lenz
Widukind Lenz was a distinguished German pediatrician, medical geneticist and dysmorphologist who was among the first to recognize the thalidomide syndrome in 1961 and alert the world to the dangers of limb and other malformations due to the mother's exposure to this drug during pregnancy.In the...
suspected a link between birth defects and the drug, a theory Lenz proved in 1961. McBride was later awarded a number of honours, including a medal and prize money by the prestigious L'Institut de la Vie in Paris.
In the United Kingdom, the drug was licensed in 1958. Of the approximately 2,000 babies born with defects, 466 survived. The drug was withdrawn in 1961. In 1968, after a long campaign by The Sunday Times
The Sunday Times
The Sunday Times is a British Sunday newspaper.The Sunday Times may also refer to:*The Sunday Times *The Sunday Times *The Sunday Times *The Sunday Times...
newspaper, a compensation settlement for the UK victims was reached with Distillers Company (now part of Diageo
Diageo
Diageo plc is a global alcoholic beverages company headquartered in London, United Kingdom. It is the world's largest producer of spirits and a major producer of beer and wine....
). This compensation, which is distributed by the Thalidomide Trust in the UK, was substantially increased by Diageo in 2005. The UK Government gave survivors a grant of £20 million, to be distributed through the Thalidomide Trust, in December 2009. In Germany approximately 2,500 thalidomide babies were born.

Frances Oldham Kelsey
Frances Kathleen Oldham Kelsey, Ph.D., M.D., is a pharmacologist, most famous as the reviewer for the U.S. Food and Drug Administration who refused to authorize thalidomide for market because she had concerns about the drug's safety. Her concerns proved to be justified when it was proven that...
refused Food and Drug Administration (FDA) approval for an application from the Richardson-Merrell
Marion Merrell Dow
Marion Merrell Dow and its predecessor Marion Laboratories was a U.S. pharmaceutical company based in Kansas City, Missouri from 1950 until 1996....
company to market thalidomide, saying further studies were needed, which reduced the impact of thalidomide in United States patients. Although thalidomide was never approved for sale in the United States, millions of tablets had been distributed to physicians during a clinical testing program. It was impossible to know how many pregnant women had been given the drug to help alleviate morning sickness or as a sedative.
Canada was the last country to stop the sales of the drug, in early 1962.
In 1962, the United States Congress
United States Congress
The United States Congress is the bicameral legislature of the federal government of the United States, consisting of the Senate and the House of Representatives. The Congress meets in the United States Capitol in Washington, D.C....
enacted laws requiring tests for safety during pregnancy before a drug can receive approval for sale in the U.S. Other countries enacted similar legislation, and thalidomide was not prescribed or sold for decades.
For correctly denying the application despite the pressure from Richardson-Merrell, Kelsey eventually received the President's Award for Distinguished Federal Civilian Service at a 1962 ceremony with President John F. Kennedy
John F. Kennedy
John Fitzgerald "Jack" Kennedy , often referred to by his initials JFK, was the 35th President of the United States, serving from 1961 until his assassination in 1963....
. In September 2010, as noted in an article titled "The Public's Quiet Savior From Harmful Medicine", the FDA honored Dr. Kelsey with the first Kelsey award. The award, given annually to a FDA staff member, came 50 years after Dr. Kelsey, then a new medical officer at the agency, first reviewed the application from the William S. Merrell Company of Cincinnati.
Teratogenic mechanism
Researchers soon discovered that only one particular optical isomer of thalidomide caused the teratogenicity. The pair of enantiomers, while mirror images of each other, cause different effects, although it is now known that the "safe" isomer can be converted to the teratogenic isomer once in the human body.Revived interest
In 1964, Jacob SheskinJacob Sheskin
Professor Jacob Sheskin, sometimes known as Sheskin Jacob was an Israeli physician best known for his 1964 discovery that thalidomide can be used as a treatment for leprosy.-Awards:* In 1975, the World Academy of Art and Science gave Prof...
, Professor at the Hebrew University of Jerusalem
Hebrew University of Jerusalem
The Hebrew University of Jerusalem ; ; abbreviated HUJI) is Israel's second-oldest university, after the Technion – Israel Institute of Technology. The Hebrew University has three campuses in Jerusalem and one in Rehovot. The world's largest Jewish studies library is located on its Edmond J...
at Hadassah University Hospital and the chief staff and manager of Hansen Leper Hospital in Jerusalem, administered thalidomide to a critically ill patient with erythema nodosum leprosum (ENL)
Erythema nodosum
Erythema nodosum is an inflammation of the fat cells under the skin characterized by tender red nodules or lumps that are usually seen on both shins...
, a painful complication of leprosy
Leprosy
Leprosy or Hansen's disease is a chronic disease caused by the bacteria Mycobacterium leprae and Mycobacterium lepromatosis. Named after physician Gerhard Armauer Hansen, leprosy is primarily a granulomatous disease of the peripheral nerves and mucosa of the upper respiratory tract; skin lesions...
, in an attempt to relieve his pain in spite of the ban. The patient slept for hours, and was able to get out of bed without aid upon awakening. The result was followed by more favorable experiences and then by a clinical trial. Sheskin found that patients with ENL, a painful skin condition, experienced pain relief when taking thalidomide.
Further work conducted in 1991 by Dr. Gilla Kaplan at Rockefeller University
Rockefeller University
The Rockefeller University is a private university offering postgraduate and postdoctoral education. It has a strong concentration in the biological sciences. It is also known for producing numerous Nobel laureates...
in New York City showed thalidomide worked in leprosy by inhibiting tumor necrosis factor alpha. Kaplan believed thalidomide could be an effective treatment for AIDS. He partnered with Celgene to further develop the potential for thalidomide in AIDS and tuberculosis. However, clinical trials for AIDS proved disappointing.
In 1994, Dr. Robert D'Amato at Harvard Medical School discovered thalidomide was a potent inhibitor of new blood vessel growth (angiogenesis). Numerous cancer clinical trials for thalidomide began based upon this finding. In 1997, Dr. Bart Barlogie reported thalidomide's initial effectiveness against multiple myeloma
Multiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
, and thalidomide was later approved in the United States by the FDA for use in this malignancy. The FDA has also approved the drug's use in the treatment of ENL. Studies are underway to determine the drug's effects on arachnoiditis
Arachnoiditis
Arachnoiditis is a neuropathic disease caused by the inflammation of the arachnoid, one of the membranes that surround and protect the nerves of the central nervous system, including the brain and spinal cord...
and several types of cancer
Cancer
Cancer , known medically as a malignant neoplasm, is a large group of different diseases, all involving unregulated cell growth. In cancer, cells divide and grow uncontrollably, forming malignant tumors, and invade nearby parts of the body. The cancer may also spread to more distant parts of the...
s. However, physicians and patients alike must go through a special process, known as STEPS, to prescribe and receive thalidomide, to ensure no more children are born with birth defects traceable to the medication. Celgene has also developed analogues to thalidomide, such as lenalidomide
Lenalidomide
Lenalidomide , initially known as CC-5013 and marketed as Revlimid by Celgene, is a derivative of thalidomide introduced in 2004....
, that are substantially more powerful and have fewer side effects — except for greater myelosuppression. Lenalidomide is now more commonly used than thalidomide for myeloma.
More recently, the World Health Organisation (WHO) has stated:
"The WHO does not recommend the use of thalidomide in leprosy as experience has shown that it is virtually impossible to develop and implement a fool-proof surveillance mechanism to combat misuse of the drug. The drug clofazimineClofazimineClofazimine is a fat-soluble riminophenazine dye used in combination with rifampicin and dapsone as multidrug therapy for the treatment of leprosy. It has been used investigationally in combination with other antimycobacterial drugs to treat Mycobacterium avium infections in AIDS patients and...
is now a component of the multidrug therapy (MDT), introduced by WHO in 1981 as the standard treatment for leprosy and now supplied free of charge to all patients worldwide."
United States
On July 16, 1998, the FDA approved the use of thalidomide for the treatment of lesions associated with erythema nodosum leprosum (ENL)Erythema nodosum
Erythema nodosum is an inflammation of the fat cells under the skin characterized by tender red nodules or lumps that are usually seen on both shins...
. Because of thalidomide's potential for causing birth defects, the drug may be distributed only under tightly controlled conditions. The FDA required that Celgene Corporation, which planned to market thalidomide under the brand name Thalomid, establish a system for thalidomide education and prescribing safety (STEPS) oversight program. The conditions required under the program include limiting prescription and dispensing rights only to authorized prescribers and pharmacies, keeping a registry of all patients prescribed thalidomide, providing extensive patient education about the risks associated with the drug, and providing periodic pregnancy tests for women who take the drug.
On May 26, 2006, the U.S. Food and Drug Administration granted accelerated approval for thalidomide (Thalomid, Celgene Corporation) in combination with dexamethasone for the treatment of newly diagnosed multiple myeloma
Multiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
(MM) patients. The FDA approval came seven years after the first reports of efficacy in the medical literature and Celgene took advantage of "off-label" marketing opportunities to promote the drug in advance of its FDA approval for the myeloma indication. Thalomid, as the drug is commercially known, sold over $300 million per year, while approved only for leprosy.
United Kingdom
Thalidomide is available to only a small number of patients in the UK, in general in specialist cancer treatment centres where research trials are taking place and where specialist doctors have experience in its use.Brazil
Brazil has the second-highest prevalence rate of leprosyLeprosy
Leprosy or Hansen's disease is a chronic disease caused by the bacteria Mycobacterium leprae and Mycobacterium lepromatosis. Named after physician Gerhard Armauer Hansen, leprosy is primarily a granulomatous disease of the peripheral nerves and mucosa of the upper respiratory tract; skin lesions...
in the world, and thalidomide has been used by Brazilian physicians as the drug of choice for the treatment of severe ENL since 1965. A study published in 1996 reported 33 people born in Brazil after 1965 with thalidomide embryopathy. Since 1994, the production, dispensing, and prescription of thalidomide have been strictly controlled, but cases of thalidomide embryopathy continue.
Possible indications
Serious infections including sepsisSepsis
Sepsis is a potentially deadly medical condition that is characterized by a whole-body inflammatory state and the presence of a known or suspected infection. The body may develop this inflammatory response by the immune system to microbes in the blood, urine, lungs, skin, or other tissues...
and tuberculosis
Tuberculosis
Tuberculosis, MTB, or TB is a common, and in many cases lethal, infectious disease caused by various strains of mycobacteria, usually Mycobacterium tuberculosis. Tuberculosis usually attacks the lungs but can also affect other parts of the body...
cause the level of tumor necrosis factor-alpha (TNFα) to rise. TNFα is a chemical mediator in the body, and may enhance the wasting process in cancer patients, as well. Thalidomide may reduce the levels of TNFα, and it is possible that the drug's effect on ENL is caused by this mechanism.
Thalidomide also has potent anti-inflammatory effects that may help ENL patients. In July 1998, the FDA approved the application of Celgene
Celgene
Celgene Corporation is a manufacturer of drug therapies for cancer and inflammatory disorders. It is incorporated in Delaware and headquartered in Summit, New Jersey...
to distribute thalidomide under the brand name Thalomid for treatment of ENL. Pharmion Corporation, who licensed the rights to market thalidomide in Europe, Australia, and various other territories from Celgene, received approval for its use against multiple myeloma
Multiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
in Australia
Australia
Australia , officially the Commonwealth of Australia, is a country in the Southern Hemisphere comprising the mainland of the Australian continent, the island of Tasmania, and numerous smaller islands in the Indian and Pacific Oceans. It is the world's sixth-largest country by total area...
and New Zealand
New Zealand
New Zealand is an island country in the south-western Pacific Ocean comprising two main landmasses and numerous smaller islands. The country is situated some east of Australia across the Tasman Sea, and roughly south of the Pacific island nations of New Caledonia, Fiji, and Tonga...
in 2003. Thalomid, in conjunction with dexamethasone
Dexamethasone
Dexamethasone is a potent synthetic member of the glucocorticoid class of steroid drugs. It acts as an anti-inflammatory and immunosuppressant...
, is now standard therapy for multiple myeloma.
Thalidomide is also prescribed for its anti-inflammatory effects in actinic prurigo
Actinic prurigo
Actinic prurigo is a not uncommon sunlight-induced, pruritic, papular or nodular skin eruption.-Causes:The cause for actinic prurigo is unknown, however researchers believe that the protein in...
, an autoimmune skin disease.
Thalidomide has been used in chronic bullous dermatosis of childhood (CBDC) with encouraging results. Peripheral neuritis may be a limiting factor for long term use of thalidomide.
Thalidomide also inhibits the growth of new blood vessels (angiogenesis
Angiogenesis
Angiogenesis is the physiological process involving the growth of new blood vessels from pre-existing vessels. Though there has been some debate over terminology, vasculogenesis is the term used for spontaneous blood-vessel formation, and intussusception is the term for the formation of new blood...
), which may be useful in treating macular degeneration
Macular degeneration
Age-related macular degeneration is a medical condition which usually affects older adults and results in a loss of vision in the center of the visual field because of damage to the retina. It occurs in “dry” and “wet” forms. It is a major cause of blindness and visual impairment in older adults...
and other diseases. This effect helps AIDS
AIDS
Acquired immune deficiency syndrome or acquired immunodeficiency syndrome is a disease of the human immune system caused by the human immunodeficiency virus...
patients with Kaposi's sarcoma
Kaposi's sarcoma
Kaposi's sarcoma is a tumor caused by Human herpesvirus 8 , also known as Kaposi's sarcoma-associated herpesvirus . It was originally described by Moritz Kaposi , a Hungarian dermatologist practicing at the University of Vienna in 1872. It became more widely known as one of the AIDS defining...
, although there are better and cheaper drugs to treat the condition. Thalidomide may be able to fight painful, debilitating aphthous lesions in the mouth and esophagus
Esophagus
The esophagus is an organ in vertebrates which consists of a muscular tube through which food passes from the pharynx to the stomach. During swallowing, food passes from the mouth through the pharynx into the esophagus and travels via peristalsis to the stomach...
of AIDS patients that prevent them from eating. The FDA formed a Thalidomide Working Group in 1994 to provide consistency between its divisions, with particular emphasis on safety monitoring. The agency also imposed severe restrictions on the distribution of Thalomid through the STEPS program.
Thalidomide is also being investigated for treating symptoms of prostate cancer
Prostate cancer
Prostate cancer is a form of cancer that develops in the prostate, a gland in the male reproductive system. Most prostate cancers are slow growing; however, there are cases of aggressive prostate cancers. The cancer cells may metastasize from the prostate to other parts of the body, particularly...
, glioblastoma, lymphoma
Lymphoma
Lymphoma is a cancer in the lymphatic cells of the immune system. Typically, lymphomas present as a solid tumor of lymphoid cells. Treatment might involve chemotherapy and in some cases radiotherapy and/or bone marrow transplantation, and can be curable depending on the histology, type, and stage...
, arachnoiditis
Arachnoiditis
Arachnoiditis is a neuropathic disease caused by the inflammation of the arachnoid, one of the membranes that surround and protect the nerves of the central nervous system, including the brain and spinal cord...
, Behçet's disease
Behçet's disease
Behçet's disease is a rare immune-mediated systemic vasculitis that often presents with mucous membrane ulceration and ocular involvements...
, and Crohn's disease
Crohn's disease
Crohn's disease, also known as regional enteritis, is a type of inflammatory bowel disease that may affect any part of the gastrointestinal tract from mouth to anus, causing a wide variety of symptoms...
. In a small trial, Australian researchers found thalidomide caused a doubling of the number of T cell
T cell
T cells or T lymphocytes belong to a group of white blood cells known as lymphocytes, and play a central role in cell-mediated immunity. They can be distinguished from other lymphocytes, such as B cells and natural killer cells , by the presence of a T cell receptor on the cell surface. They are...
s in patients, allowing the patients' own immune system
Immune system
An immune system is a system of biological structures and processes within an organism that protects against disease by identifying and killing pathogens and tumor cells. It detects a wide variety of agents, from viruses to parasitic worms, and needs to distinguish them from the organism's own...
to attack cancer cells.
Studies carried out in animal models have suggested the use of combined therapy with thalidomide and glucantime could have a therapeutic benefit in the treatment of visceral leshmaniasis.
A study published in April 2010 discussed the ability of thalidomide to induce vessel maturation, which may be useful as a therapeutic strategy for the treatment of vascular malformations. The research was conducted in an experimental model of the genetic disease hereditary hemorrhagic telangiectasia
Hereditary hemorrhagic telangiectasia
Hereditary hemorrhagic telangiectasia , also known as Osler-Weber-Rendu disease and Osler-Weber-Rendu syndrome, is a genetic disorder that leads to abnormal blood vessel formation in the skin, mucous membranes, and often in organs such as the lungs, liver and brain.It may lead to nosebleeds, acute...
.
Thalidomide and multiple myeloma
Thalidomide was first tested in humans as a single agent for the treatment of multiple myelomaMultiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
in 1996 due to its antiangiogenic activity. The New England Journal of Medicine published the full study in 1999. Since then, many studies have shown that thalidomide, in combination with dexamethasone
Dexamethasone
Dexamethasone is a potent synthetic member of the glucocorticoid class of steroid drugs. It acts as an anti-inflammatory and immunosuppressant...
, has increased the survival of multiple myeloma patients. The combination of thalidomide and dexamethasone, often in combination with melphalan
Melphalan
Melphalan hydrochloride is a chemotherapy drug belonging to the class of nitrogen mustard alkylating agents.An alkylating agent adds an alkyl group to DNA...
, is now one of the most common regimens for patients with newly diagnosed multiple myeloma, with an improved response rate of up to 60-70%. Thalidomide may also cause side effects, such as polyneuropathy
Polyneuropathy
Polyneuropathy is a neurological disorder that occurs when many peripheral nerves throughout the body malfunction simultaneously. It may be acute and appear without warning, or chronic and develop gradually over a longer period of time. Many polyneuropathies have both motor and sensory...
, fatigue, skin rash, and venous thromboembolism (VTE), or blood clots, which could lead to stroke
Stroke
A stroke, previously known medically as a cerebrovascular accident , is the rapidly developing loss of brain function due to disturbance in the blood supply to the brain. This can be due to ischemia caused by blockage , or a hemorrhage...
or myocardial infarction
Myocardial infarction
Myocardial infarction or acute myocardial infarction , commonly known as a heart attack, results from the interruption of blood supply to a part of the heart, causing heart cells to die...
. Bennett et al. have conducted a systematic review of VTE associated with thalidomide in multiple myeloma patients. They have found that when thalidomide was administered without prophylaxis, VTE rates reached as high as 26%. Owing to the high rates of VTE associated with thalidomide in combination with dexamethasone or doxorubicin
Doxorubicin
Doxorubicin INN is a drug used in cancer chemotherapy. It is an anthracycline antibiotic, closely related to the natural product daunomycin, and like all anthracyclines, it works by intercalating DNA....
, a black box warning
Black box warning
In the United States, a black box warning is a type of warning that appears on the package insert for prescription drugs that may cause serious adverse effects...
was added in the US in 2006 to the package insert for thalidomide, indicating that patients with multiple myeloma who receive thalidomide-dexamethasone may benefit from concurrent thromboembolism prophylaxis or aspirin
Aspirin
Aspirin , also known as acetylsalicylic acid , is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It was discovered by Arthur Eichengrun, a chemist with the German company Bayer...
. In addition, owing to these side effects, newer drugs, such as bortezomib
Bortezomib
Bortezomib is the first therapeutic proteasome inhibitor to be tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma and mantle cell lymphoma...
(marketed as Velcade) and a thalidomide derivative, lenalidomide
Lenalidomide
Lenalidomide , initially known as CC-5013 and marketed as Revlimid by Celgene, is a derivative of thalidomide introduced in 2004....
(marketed as Revlimid), have increased in popularity.
Teratogenic mechanism

Racemic
In chemistry, a racemic mixture, or racemate , is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule. The first known racemic mixture was "racemic acid", which Louis Pasteur found to be a mixture of the two enantiomeric isomers of tartaric acid.- Nomenclature :A...
– it contains both left- and right-handed isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s in equal amounts. The (R) enantiomer is effective against morning sickness, but the (S) is teratogenic
Teratology
Teratology is the study of abnormalities of physiological development. It is often thought of as the study of human birth defects, but it is much broader than that, taking in other non-birth developmental stages, including puberty; and other non-human life forms, including plants.- Etymology :The...
. The enantiomers can interconvert (racemize) in vivo
In vivo
In vivo is experimentation using a whole, living organism as opposed to a partial or dead organism, or an in vitro controlled environment. Animal testing and clinical trials are two forms of in vivo research...
– that is, if a human is given pure (R)-thalidomide or (S)-thalidomide, both isomers will later be found in the serum
Blood plasma
Blood plasma is the straw-colored liquid component of blood in which the blood cells in whole blood are normally suspended. It makes up about 55% of the total blood volume. It is the intravascular fluid part of extracellular fluid...
– therefore, administering only one enantiomer will not prevent the teratogenic effect.
The mechanism of thalidomide's teratogenic action has led to over 2000 research papers and the proposal of 15 or 16 plausible mechanisms. A theoretical synthesis in 2000 suggested the following mechanism: thalidomide intercalates
Intercalation (chemistry)
In chemistry, intercalation is the reversible inclusion of a molecule between two other molecules . Examples include DNA intercalation and graphite intercalation compounds.- DNA intercalation :...
(inserts itself) into DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
in guanine
Guanine
Guanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine . In DNA, guanine is paired with cytosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with...
-cytosine
Cytosine
Cytosine is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine . It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached . The nucleoside of cytosine is cytidine...
-rich regions. Owing to its glutarimide part, (S) thalidomide fits neatly into the major groove of DNA at purine sites. Such intercalation impacts upon the promoter regions of the genes controlling the development of limbs, ears, and eyes, such as IGF-I and FGF-2. These normally activate the production of the cell surface attachment integrin
Integrin
Integrins are receptors that mediate attachment between a cell and the tissues surrounding it, which may be other cells or the ECM. They also play a role in cell signaling and thereby regulate cellular shape, motility, and the cell cycle....
αvβ3, with the resulting αvβ3 integrin dimer stimulating angiogenesis
Angiogenesis
Angiogenesis is the physiological process involving the growth of new blood vessels from pre-existing vessels. Though there has been some debate over terminology, vasculogenesis is the term used for spontaneous blood-vessel formation, and intussusception is the term for the formation of new blood...
in developing limb buds. This then promotes the outgrowth of the bud (IGF-I and FGF-2 are also both known to stimulate angiogenesis). Therefore, by inhibiting the chain of events, thalidomide causes the truncation of limb development. In 2009, this theory received strong support, with research showing "conclusively that loss of newly formed blood vessels is the primary cause of thalidomide teratogenesis, and developing limbs are particularly susceptible because of their relatively immature, highly angiogenic vessel network."
Inactivation of the protein cereblon
Thalidomide binds to and inactivates the protein cereblonCereblon
Cereblon is a protein that in humans is encoded by the CRBN gene. The gene that encodes the cereblon protein is found on the human chromosome 3, on the short arm at position p26.3 from base pair 3,190,676 to base pair 3,221,394...
, which is important in limb formation. The inactivation leads to a teratogenic effect on fetal development
Fetal development
Prenatal or antenatal development is the process in which a human embryo or fetus gestates during pregnancy, from fertilization until birth. Often, the terms fetal development, foetal development, or embryology are used in a similar sense.After fertilization the embryogenesis starts...
. This was confirmed when the scientists, using genetic techniques, reduced the production of cereblon
Cereblon
Cereblon is a protein that in humans is encoded by the CRBN gene. The gene that encodes the cereblon protein is found on the human chromosome 3, on the short arm at position p26.3 from base pair 3,190,676 to base pair 3,221,394...
in developing chick and zebrafish embryos. These embryos had defects similar to those treated with thalidomide. While the mechanism that causes teratogenicity has been established, the mechanism for other therapeutic effects remains unclear.
Mechanism in multiple myeloma
Thalidomide appears to inhibit the disease progression in multiple myelomaMultiple myeloma
Multiple myeloma , also known as plasma cell myeloma or Kahler's disease , is a cancer of plasma cells, a type of white blood cell normally responsible for the production of antibodies...
by several mechanisms, as resulting mainly from experiments on myeloma cancer cell lines:
- Inhibition of the production of interleukin-6 (IL-6), which is a growth factor for the proliferation of myeloma cells
- Activation of apoptotic pathways through caspase 8Caspase 8Caspase 8 is a caspase protein, encoded by the CASP8 gene. It most likely acts upon caspase 3.CASP8 orthologs have been identified in numerous mammals for which complete genome data are available...
-mediated cell death - At the mitochondrial level, thalidomide results in induction of c-jun terminal kinase (JNK)-dependent release of cytochrome-c and SmacDiablo homologDiablo homolog, mitochondrial is a protein that in humans is encoded by the DIABLO gene . DIABLO is also referred to as second mitochondria-derived activator of caspases or SMAC.- Function :...
into the cytosol of cells, affecting apoptosis. - Activation of T cells to produce IL-2, thereby altering the amount and function of natural killer cellNatural killer cellNatural killer cells are a type of cytotoxic lymphocyte that constitute a major component of the innate immune system. NK cells play a major role in the rejection of tumors and cells infected by viruses...
s (NK cells), thus augmenting the activity of NK-dependent cytotoxicity
Thalidomide analogs
The exploration of the antiangiogenic and immunomodulatory activities of thalidomide has led to the study and creation of thalidomide analogAnalog (chemistry)
In chemistry, a structural analog , also known as chemical analog or simply analog, is a compound having a structure similar to that of another one, but differing from it in respect of a certain component. It can differ in one or more atoms, functional groups, or substructures, which are replaced...
s. In 2005, Celgene received FDA approval for lenalidomide
Lenalidomide
Lenalidomide , initially known as CC-5013 and marketed as Revlimid by Celgene, is a derivative of thalidomide introduced in 2004....
(Revlimid) as the first commercially useful derivative. Revlimid is available only in a restricted distribution setting to avoid its use during pregnancy. Further studies are being conducted to find safer compounds with useful qualities. Another analog, pomalidomide
Pomalidomide
Pomalidomide , is a derivative of thalidomide and acts as an immunomodulator. It can be taken orally.-Clinical trials:Phase I trial results showed neutropenia was a frequent side effect....
, is in the clinical trial phase. These thalidomide analogs can be used to treat different diseases, or used in a regimen to fight two conditions.
Notable people affected
- Rock Brynner, son of Yul BrynnerYul BrynnerYul Brynner was a Russian-born actor of stage and film. He was best known for his portrayal of Mongkut, king of Siam, in the Rodgers and Hammerstein musical The King and I, for which he won an Academy Award for Best Actor for the film version; he also played the role more than 4,500 times on...
, author of Dark Remedy, who took Thalidomide as an adult for his immune disorder - Mat FraserMat FraserMat Fraser is an English rock musician, actor and performance artist. Between 1980 and 1995 he was a drummer with several rock bands including Fear of Sex, The Reasonable Strollers, Joyride, The Grateful Dub, and Living in Texas, the latter of which had a number one single in Italy.- Life :Fraser...
, musician, actor and performance artist born with phocomeliaPhocomeliaPhocomelia is an extremely rare congenital disorder involving the limbs . Étienne Geoffroy Saint-Hilaire coined the term in 1836....
of both arms - Alvin LawAlvin LawAlvin Law is a motivational speaker and former radio broadcaster.Law was born without arms as a consequence of his mother's use of thalidomide while pregnant...
, radio broadcaster, born without arms - Louise Medus Mansell, daughter of David Mason, campaigner for increased compensation for thalidomide children, born with no arms or legs
- Tony MeléndezTony MelendezJosé Antonio Meléndez Rodríguez is a Nicaraguan American guitar player, composer and singer and songwriter who was born without arms. His mother took Thalidomide while pregnant, which caused his disability. Meléndez has learned to play the guitar with his feet.-Career:Meléndez began playing and...
, award winning singer and guitarist who plays with his feet, is known internationally due to the recognition received from Pope John Paul IIPope John Paul IIBlessed Pope John Paul II , born Karol Józef Wojtyła , reigned as Pope of the Catholic Church and Sovereign of Vatican City from 16 October 1978 until his death on 2 April 2005, at of age. His was the second-longest documented pontificate, which lasted ; only Pope Pius IX ...
and U.S. President Ronald ReaganRonald ReaganRonald Wilson Reagan was the 40th President of the United States , the 33rd Governor of California and, prior to that, a radio, film and television actor....
. - Thomas QuasthoffThomas QuasthoffThomas Quasthoff is a German bass-baritone. Although his reputation was initially based on his performance of Romantic lieder, Quasthoff has proven to have a remarkable range from the Baroque cantatas of Bach to solo jazz improvisations.-Biography:Quasthoff was born in Hildesheim, Germany, with...
, an internationally acclaimed bass-baritone, who describes himself: "1.34 meters tall, short arms, seven fingers — four right, three left — large, relatively well-formed head, brown eyes, distinctive lips; profession: singer" - Niko von GlasowNiko von GlasowNiko von Glasow is a feature film director as well as a documentary film director. Von Glasow is the son of Ernst Brücher and Majella Neven DuMont, founders of the DuMont Publishing house in Cologne....
produced a documentary called Nobody's Perfect, based on the lives of 12 people affected by the drug, which was released in 2008. - Terry WilesTerry WilesTerrence 'Terry' Wiles was one of the most disabled thalidomide babies born in the UK. He has since become known internationally through the television drama On Giant's Shoulders and the best-selling book of the same name....
, born with phocomelia of both arms and legs, has become known internationally through the television drama On Giant's Shoulders and the best-selling book of the same name. - A 2007 fiction book Thalidomide Kid by author Kate Rigby described a story about a boy born with no arms confronting pain and prejudice during the 1970s.
External links
- Thalidomide monograph from Chemical and Engineering News. (Archived by WebCite® at http://www.webcitation.org/5nWHyOCfI)
- Thalidomide product monograph (Needs registration)
- Multiple Myeloma Research Foundation article on Thalidomide
- International Myeloma Foundation article on Thalidomide
- Thalidomide — Annotated List of Links (covering English and German pages)
- WHO Pharmaceuticals Newsletter No. 2, 2003 - See page 11, Feature Article
- Grünenthal GmbH — Thalidomide
- Celgene website on Thalomid
- The Return of Thalidomide — BBC
- CBC Digital Archives – Thalidomide: Bitter Pills, Broken Promises
- Thalidomide UK
- The Thalidomide Trust
- The International Contergan Thalidomide Alliance website
- "The Big Pitch: How would you conduct a campaign for the new Thalidomide Drugs?", forum of pharmaceutical and medical marketing professionals commenting on how they would address the thalidomine controversies.

