
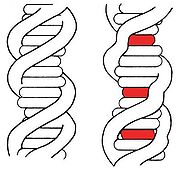
Intercalation (chemistry)
Encyclopedia
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, intercalation is the reversible inclusion of a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
(or group) between two other molecules (or groups). Examples include DNA intercalation and graphite intercalation compound
Graphite intercalation compound
Graphite intercalation compounds are complex materials having formula XCy where element or molecule X is inserted between the graphite layers. In this type of compound, the graphite layers remain largely intact and the guest molecules or atoms are located in between...
s.
DNA intercalation
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. In a narrower sense, it is a signal triggering molecule, binding to a site on a target protein.The binding occurs by intermolecular forces, such as ionic bonds, hydrogen...
s) can interact with DNA. Ligands may interact with DNA by covalently binding, electrostatically binding, or intercalating. Intercalation occurs when ligands of an appropriate size and chemical nature fit themselves in between base pairs of DNA. These ligands are mostly polycyclic, aromatic, and planar, and therefore often make good nucleic acid stains
Staining (biology)
Staining is an auxiliary technique used in microscopy to enhance contrast in the microscopic image. Stains and dyes are frequently used in biology and medicine to highlight structures in biological tissues for viewing, often with the aid of different microscopes...
. Intensively studied DNA intercalators include berberine
Berberine
Berberine is a quaternary ammonium salt from the protoberberine group of isoquinoline alkaloids. It is found in such plants as Berberis Berberine is a quaternary ammonium salt from the protoberberine group of isoquinoline alkaloids. It is found in such plants as Berberis Berberine is a quaternary...
, ethidium bromide
Ethidium bromide
Ethidium bromide is an intercalating agent commonly used as a fluorescent tag in molecular biology laboratories for techniques such as agarose gel electrophoresis. It is commonly abbreviated as "EtBr", which is also an abbreviation for bromoethane...
, proflavine
Proflavine
Proflavine , also called proflavin and diaminoacridine, is an acriflavine derivative, a disinfectant bacteriostatic against many gram-positive bacteria...
, daunomycin, doxorubicin
Doxorubicin
Doxorubicin INN is a drug used in cancer chemotherapy. It is an anthracycline antibiotic, closely related to the natural product daunomycin, and like all anthracyclines, it works by intercalating DNA....
, and thalidomide
Thalidomide
Thalidomide was introduced as a sedative drug in the late 1950s that was typically used to cure morning sickness. In 1961, it was withdrawn due to teratogenicity and neuropathy. There is now a growing clinical interest in thalidomide, and it is introduced as an immunomodulatory agent used...
. DNA intercalators are used in chemotherapeutic
Chemotherapy
Chemotherapy is the treatment of cancer with an antineoplastic drug or with a combination of such drugs into a standardized treatment regimen....
treatment to inhibit DNA replication in rapidly growing cancer cells. Examples include doxorubicin (adriamycin) and daunorubicin (both of which are used in treatment of Hodgkin's lymphoma), and dactinomycin (used in Wilm's tumour, Ewing's Sarcoma, rhabdomyosarcoma).
In order for an intercalator to fit between base pairs, the DNA must dynamically open a space between its base pairs by unwinding. The degree of unwinding varies depending on the intercalator; for example, ethidium cation (the ionic form of ethidium bromide
Ethidium bromide
Ethidium bromide is an intercalating agent commonly used as a fluorescent tag in molecular biology laboratories for techniques such as agarose gel electrophoresis. It is commonly abbreviated as "EtBr", which is also an abbreviation for bromoethane...
found in aqueous solution) unwinds DNA by about 26°, whereas proflavine unwinds it by about 17°. This unwinding causes the base pairs to separate, or "rise", creating an opening of about 0.34 nm (3.4 Å). This unwinding induces local structural changes to the DNA strand, such as lengthening of the DNA strand or twisting of the base pairs. These structural modifications can lead to functional changes, often to the inhibition of transcription
Transcription (genetics)
Transcription is the process of creating a complementary RNA copy of a sequence of DNA. Both RNA and DNA are nucleic acids, which use base pairs of nucleotides as a complementary language that can be converted back and forth from DNA to RNA by the action of the correct enzymes...
and replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
and DNA repair processes, which makes intercalators potent mutagen
Mutagen
In genetics, a mutagen is a physical or chemical agent that changes the genetic material, usually DNA, of an organism and thus increases the frequency of mutations above the natural background level. As many mutations cause cancer, mutagens are therefore also likely to be carcinogens...
s. For this reason, DNA intercalators are often carcinogen
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes...
ic, such as the exo
Endo-exo isomerism
Endo-exo isomerism is a special type of isomerism found in organic compounds with a substituent on a bridged ring system. The prefix endo is reserved for the isomer with the substituent located closest, or "syn," to the longest bridge. The prefix exo is reserved for the isomer with the substituent...
(but not the endo) 8,9 epoxide
Epoxide
An epoxide is a cyclic ether with three ring atoms. This ring approximately defines an equilateral triangle, which makes it highly strained. The strained ring makes epoxides more reactive than other ethers. Simple epoxides are named from the parent compound ethylene oxide or oxirane, such as in...
of aflatoxin
Aflatoxin
Aflatoxins are naturally occurring mycotoxins that are produced by many species of Aspergillus, a fungus, the most notable ones being Aspergillus flavus and Aspergillus parasiticus. Aflatoxins are toxic and among the most carcinogenic substances known...
B1, acridine
Acridine
Acridine, C13H9N, is an organic compound and a nitrogen heterocycle. Acridine is also used to describe compounds containing the C13N tricycle....
s such as proflavine
Proflavine
Proflavine , also called proflavin and diaminoacridine, is an acriflavine derivative, a disinfectant bacteriostatic against many gram-positive bacteria...
or quinacrine
Quinacrine
Quinacrine is a drug with a number of different medical applications. It is related to mefloquine.-Uses:Its main effects are as an antiprotozoal, antirheumatic and an intrapleural sclerosing agent....
, or ethidium bromide.
Intercalation as a mechanism of interaction between cationic, planar, polycyclic aromatic systems of the correct size (on the order of a base pair) was first proposed by Leonard Lerman
Leonard Lerman
Leonard Lerman is an American scientist most noted for his work on DNA.As a graduate student with Linus Pauling at the California Institute of Technology, Lerman discovered that antibodies have two binding sites. Later, perhaps his most important discovery was that certain molecules bind to DNA by...
in 1961. One proposed mechanism of intercalation is as follows: In aqueous isotonic solution, the cationic intercalator is attracted electrostatically to the polyanionic DNA. The ligand displaces a sodium and/or magnesium cation that always surrounds DNA (to balance its charge), forming a weak electrostatic bond with the outer surface of DNA. From this position, the ligand may then slide into the hydrophobic environment found between the base pairs and away from the hydrophilic outer environment surrounding the DNA. The base pairs transiently form such openings due to energy absorbed during collisions with solvent molecules.
Materials science
Many layered solids intercalate guest molecules. A famous example is the intercalation of potassium into graphiteGraphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
. Intercalation expands the "van der Waals gap" between sheets, which requires energy. Usually this energy is supplied by charge transfer between the guest and the host solid, i.e., redox. Aside from graphite, well-known intercalation hosts are the layered dichalcogenides such as tantalum disulfide and iron oxychloride
Iron oxychloride
Iron oxychloride is the inorganic compound with the formula FeOCl. This purple solid adopts a layered structure, akin to that of cadmium chloride. The material slowly hydrolyses in moist air. The solid intercalates electron-donors such as tetrathiafulvalene and even pyridine to give mixed...
. In characteristic manner, intercalation is analyzed by X-ray diffraction, since the spacing between sheets increases, and by electrical conductivity, since charge transfer alters the number of charge carriers.
See also
- Stacking (chemistry)Stacking (chemistry)In chemistry, pi stacking refers to attractive, noncovalent interactions between aromatic rings. These interactions are historically thought to be important in to base stacking of DNA nucleotides, protein folding, template-directed synthesis, materials science, and molecular recognition, although...
- Molecular tweezers
- Graphite intercalation compoundGraphite intercalation compoundGraphite intercalation compounds are complex materials having formula XCy where element or molecule X is inserted between the graphite layers. In this type of compound, the graphite layers remain largely intact and the guest molecules or atoms are located in between...

