
Catenane
Encyclopedia
Mechanically-interlocked molecular architectures
Mechanically interlocked molecular architectures are connections of molecules not through traditional bonds, but instead as a consequence of their topology. This connection of molecules is analogous to keys on a key chain loop. The keys are not directly connected to the key chain loop but they...
consisting of two or more interlocked macrocycle
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
s. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. Catenane is derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
catena meaning "chain". They are conceptually related to other mechanically-interlocked molecular architectures, such as rotaxane
Rotaxane
A rotaxane is a mechanically-interlocked molecular architecture consisting of a "dumbbell shaped molecule" which is threaded through a "macrocycle" . The name is derived from the Latin for wheel and axle...
s, molecular knots or molecular Borromean rings
Molecular Borromean rings
Molecular Borromean rings are an example of a mechanically-interlocked molecular architecture in which three macrocycles are interlocked in such a way that breaking any macrocycle allows the others to disassociate. They are the smallest examples of Borromean rings. The synthesis of molecular...
. Recently the terminology "mechanical bond
Mechanical bond
The mechanical bond is a type of chemical bond found in mechanically-interlocked molecular architectures such as catenanes and rotaxanes. Unlike classical molecular structures, interlocked molecules consist of two or more separate components which are not connected by chemical bonds...
" has been coined that describes the connection between the macrocycles of a catenane.
Synthesis
There are two primary approaches to the organic synthesisOrganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to the first successful synthesis of a catenane; however, the method is highly inefficient, requiring high dilution of the "closing" ring and a large excess of the pre-formed ring, and is rarely used.
The second approach relies on supramolecular
Supramolecular chemistry
Supramolecular chemistry refers to the area of chemistry beyond the molecules and focuses on the chemical systems made up of a discrete number of assembled molecular subunits or components...
preorganization of the macrocyclic precursors utilizing hydrogen bonding
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
, metal coordination, hydrophobic forces
Hydrophobic effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in aqueous solution and exclude water molecules. The name, literally meaning "water-fearing," describes the segregation and apparent repulsion between water and nonpolar substances...
, or coulombic interactions. These non-covalent interactions offset some of the entropic
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
cost of association and help position the components to form the desired catenane upon the final ring-closing. This "template-directed" approach, together with the use of high-pressure conditions, can provide yields of over 90%, thus improving the potential of catenanes for applications. An example of this approach used bis-bipyridinium
Pyridinium
Pyridinium refers to the cationic form of pyridine. This can either be due to protonation of the ring nitrogen or because of addition of a substituent to the ring nitrogen, typically via alkylation. The lone pair of electrons on the nitrogen atom of pyridine is not delocalized, and thus pyridine...
salts which form strong complexes threaded through crown ether bis(para-phenylene)-34-crown-10. Sanders
Jeremy Sanders
Jeremy Keith Morris Sanders, is a British chemist who is known for his contributions to many fields including NMR spectroscopy and supramolecular chemistry. He has been Head of the School of Physical Sciences at the University of Cambridge since 2009; he was also Deputy Vice-Chancellor 2006–2010,...
has shown that dynamic combinatorial approaches using reversible chemistry can be particularly successful in preparing new catenanes of unpredictable structure.
Properties and applications
A particularly interesting property of many catenanes is the ability of the rings to rotate with respect to one another. This motion can often be detected and measured by NMR spectroscopyNMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
, among other methods. When molecular recognition motifs exist in the finished catenane (usually those that were used to synthesize the catenane), the catenane can have one or more thermodynamically
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
preferred positions of the rings with respect to each other. In the case where one recognition site is a switchable moiety, a mechanical molecular switch
Molecular switch
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to changes in e.g. pH, light, temperature, an electrical current, microenvironment, or the presence of a ligand. In some cases, a...
results. When a catenane is synthesized by coordination of the macrocycles around a metal ion, then removal and re-insertion of the metal ion can switch the free motion of the rings on and off.
Catenanes have been synthesized incorporating many functional units, including redox-active groups (e.g. viologen
Viologen
Viologens are toxic bipyridinium derivatives of 4,4'-bipyridyl. The name is because this class of compounds is easily reduced to the radical mono cation, which is intensely blue coloured....
, TTF=tetrathiafulvalene
Tetrathiafulvalene
Tetrathiafulvalene is a organosulfur compound with the formula 2. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, 2, by replacement of four CH groups with sulfur atoms...
), photoisomerizable groups (e.g. azobenzene
Azobenzene
Azobenzene is a chemical compound composed of two phenyl rings linked by a N=N double bond. It is the best known example of an azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of molecules that share the core azobenzene structure, with different chemical...
), fluorescent groups and chiral
Chirality (chemistry)
A chiral molecule is a type of molecule that lacks an internal plane of symmetry and thus has a non-superimposable mirror image. The feature that is most often the cause of chirality in molecules is the presence of an asymmetric carbon atom....
groups. Some such units have been used to create molecular switches as described above, as well as for the fabrication of molecular electronic
Molecular electronics
Molecular electronics, sometimes called moletronics, involves the study and application of molecular building blocks for the fabrication of electronic components...
devices and molecular sensor
Molecular sensor
A molecular sensor or chemosensor is a molecule that interacts with an analyte to produce a detectable change. Molecular sensors combine molecular recognition with some form of reporter so the presence of the guest can be observed...
s.
Families of catenanes
There are a number of distinct methods of holding the precursors together prior to the ultimate ring-closing reaction in a template-directed catenane synthesis. Each noncovalent approach to catenane formation results in what can be considered different families of catenanes.Another family of catenanes are called pretzelanes or bridged [2]catenanes after their likeness to pretzel
Pretzel
A pretzel is a type of baked food made from dough in soft and hard varieties and savory or sweet flavors in a unique knot-like shape, originating in Europe...
s with a spacer linking the two macrocycles. In one such system one macrocycle is an electron deficient oligo Bis-bipyridinium
Pyridinium
Pyridinium refers to the cationic form of pyridine. This can either be due to protonation of the ring nitrogen or because of addition of a substituent to the ring nitrogen, typically via alkylation. The lone pair of electrons on the nitrogen atom of pyridine is not delocalized, and thus pyridine...
ring and the other cycle is crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
cyclophane
Cyclophane
A cyclophane is a hydrocarbon consisting of an aromatic unit and an aliphatic chain that forms a bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known...
based on para phenylene
Poly(p-phenylene)
Poly is the precursor to a conducting polymer of the rigid-rod polymer host family. Oxidation or the use of dopants is used to convert the non-conductive form to a semiconductor. It is made of repeating p-phenylene units....
or naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
. X-ray diffraction shows that due to pi-pi interactions the aromatic group of the cyclophane is held firmly inside the pyridinium ring. A limited number of (rapidly-interchanging) conformers exist for this type of compound.
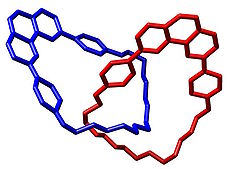
In handcuff-shaped catenanes , two connected rings are threaded through the same ring. The bis-macrocycle (red) contains two phenanthroline
Phenanthroline
Phenanthroline is a heterocyclic organic compound. As a bidentate ligand in coordination chemistry, it forms strong complexes with most metal ions...
units in a crown ether
Crown ether
Crown ethers are cyclic chemical compounds that consist of a ring containing several ether groups. The most common crown ethers are oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., -CH2CH2O-. Important members of this series are the tetramer , the pentamer , and the hexamer...
chain. The interlocking ring is self-assembled
Molecular self-assembly
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly, intramolecular self-assembly and intermolecular self-assembly...
when two more phenanthroline units with alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
arms coordinate through a copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
(I) complex followed by a metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
ring closing step.

