
Hydrophobic effect
Encyclopedia

Aqueous solution
An aqueous solution is a solution in which the solvent is water. It is usually shown in chemical equations by appending aq to the relevant formula, such as NaCl. The word aqueous means pertaining to, related to, similar to, or dissolved in water...
and exclude water molecules. The name, literally meaning "water-fearing," describes the segregation
Segregation in materials
Segregation in Materials refers to the enrichment of a material constituent at a free surface or an internal interface of a material. In a polycrystalline solid, a segregation site can be a dislocation, grain boundary, stacking fault, or an interface with a precipitate or secondary phase within the...
and apparent repulsion between water and nonpolar substances. The hydrophobic effect explains the separation of a mixture of oil and water into its two components, and the beading of water on nonpolar surfaces such as waxy leaves. At the molecular level, the hydrophobic effect is important in driving protein folding
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
, formation of lipid bilayer
Lipid bilayer
The lipid bilayer is a thin membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around cells. The cell membrane of almost all living organisms and many viruses are made of a lipid bilayer, as are the membranes surrounding the cell nucleus...
s and micelle
Micelle
A micelle is an aggregate of surfactant molecules dispersed in a liquid colloid. A typical micelle in aqueous solution forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single tail regions in the micelle centre. This phase is...
s, insertion of membrane protein
Membrane protein
A membrane protein is a protein molecule that is attached to, or associated with the membrane of a cell or an organelle. More than half of all proteins interact with membranes.-Function:...
s into the nonpolar lipid environment and protein-small molecule
Small molecule
In the fields of pharmacology and biochemistry, a small molecule is a low molecular weight organic compound which is by definition not a polymer...
interactions. Substances for which this effect is observed are known as hydrophobe
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
s.
Amphiphiles
AmphiphilesAmphiphiles
Amphiphile is a term describing a chemical compound possessing both hydrophilic and lipophilic properties...
are molecules that have both hydrophobic and hydrophilic domains. Detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s are composed of amphiphiles that allow hydrophobic molecules to be solubilized in water by forming micelle
Micelle
A micelle is an aggregate of surfactant molecules dispersed in a liquid colloid. A typical micelle in aqueous solution forms an aggregate with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single tail regions in the micelle centre. This phase is...
s and bilayers (as in soap bubbles). They are also important to cell membranes composed of amphiphilic phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
s that prevent the internal aqueous environment of a cell from mixing with external water.
Folding of macromolecules
In the case of protein foldingProtein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
, the hydrophobic effect is important to understand the structure of proteins that have hydrophobic amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, such as alanine
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
, valine
Valine
Valine is an α-amino acid with the chemical formula HO2CCHCH2. L-Valine is one of 20 proteinogenic amino acids. Its codons are GUU, GUC, GUA, and GUG. This essential amino acid is classified as nonpolar...
, leucine
Leucine
Leucine is a branched-chain α-amino acid with the chemical formula HO2CCHCH2CH2. Leucine is classified as a hydrophobic amino acid due to its aliphatic isobutyl side chain. It is encoded by six codons and is a major component of the subunits in ferritin, astacin and other 'buffer' proteins...
, isoleucine
Isoleucine
Isoleucine is an α-amino acid with the chemical formula HO2CCHCHCH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA....
, phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
, tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
and methionine
Methionine
Methionine is an α-amino acid with the chemical formula HO2CCHCH2CH2SCH3. This essential amino acid is classified as nonpolar. This amino-acid is coded by the codon AUG, also known as the initiation codon, since it indicates mRNA's coding region where translation into protein...
clustered together within the protein. Structures of water-soluble proteins have a hydrophobic core in which side chains are buried from water, which stabilizes the folded state, and charged and polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
side chains are situated on the solvent-exposed surface where they interact with surrounding water molecules. Minimizing the number of hydrophobic side chains exposed to water is the principal driving force behind the folding process, although formation of hydrogen bonds within the protein also stabilizes protein structure.
The energetics of DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
tertiary structure assembly were determined to be driven by the hydrophobic effect, in addition to Watson-Crick base pairing (which is responsible for sequence selectivity) and a significant contribution from stacking interactions
Stacking (chemistry)
In chemistry, pi stacking refers to attractive, noncovalent interactions between aromatic rings. These interactions are historically thought to be important in to base stacking of DNA nucleotides, protein folding, template-directed synthesis, materials science, and molecular recognition, although...
between the aromatic bases.
Protein purification
In biochemistryBiochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
, the hydrophobic effect can be used to separate mixtures of proteins based on their hydrophobicity. Column chromatography
Column chromatography
Column chromatography in chemistry is a method used to purify individual chemical compounds from mixtures of compounds. It is often used for preparative applications on scales from micrograms up to kilograms.The main advantage of column chromatography is the relatively low cost and disposability...
with a hydrophobic stationary phase such as phenyl-sepharose
Sepharose
Sepharose is a tradename for a crosslinked, beaded-form of a polysaccharide polymer material extracted from seaweed. It is crosslinked through lysine side chains. Iodoacetyl functional groups can be added to selectively bind cysteine side chains and this method is often used to immobilize peptides....
will cause more hydrophobic proteins to travel more slowly, while less hydrophobic ones elute from the column sooner. To achieve better separation, a salt may be added (higher concentrations of salt increase the hydrophobic effect) and its concentration decreased as the separation goes on.
The origin of hydrophobic effect
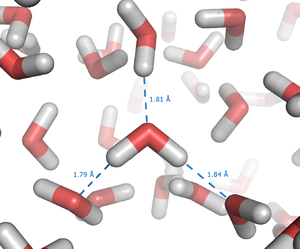
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
effect originating from the disruption of highly dynamic hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s between molecules of liquid water by the nonpolar solute. Because a purely hydrocarbon molecule or region is incapable of forming hydrogen bonds with water, introduction of the hydrocarbon into water causes disruption of the hydrogen bonding network between water molecules. The hydrogen bonds are partially reconstructed by building a water "cage" around the hydrocarbon molecule or hydrophpic molecular groups, but the water molecules that form the "cage" (or solvation shell
Solvation shell
A Solvation shell is a shell of any chemical species acting as a solvent, surrounding a solute species. When the solvent is water it is often referred to as a hydration shell or hydration sphere....
) have restricted mobilities. In the solvation shell of small nonpolar particles, the restriction amounts to some 10%, e.g. in the case of dissolved Xe at room temperature, a mobility restriction of 30% has been found. In the case of larger nonpolar molecules the reorientational and translational motion of the water molecules in the solvation shell may be restricted by a factor of two to four. Thus at 25°C the reorientational correlation time of water increases from 2 to 4-8 picoseconds. Generally, this leads to significant losses in translational and rotational entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
of water molecules and makes the process unfavorable in terms of free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of the system. This unfavorable free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
is directly proportional to the number of carbon-hydrogen bonds in the hydrocarbon molecule. By aggregating together, nonpolar molecules reduce the surface area exposed to water and minimize their disruptive effect.
The hydrophobic effect can be quantified by measuring the partition coefficient
Partition coefficient
In chemistry and the pharmaceutical sciences, a partition- or distribution coefficient is the ratio of concentrations of a compound in the two phases of a mixture of two immiscible solvents at equilibrium. The terms "gas/liquid partition coefficient" and "air/water partition coefficient" are...
s of non-polar molecules between water and non-polar solvents. The partition coefficients can be transformed to free energy
Gibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of transfer which includes enthalpic
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
and entropic components, ΔG = ΔH - TΔS. These components are experimentally determined by calorimetry
Differential scanning calorimetry
Differential scanning calorimetry or DSC is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature...
. The hydrophobic effect was found to be entropy-driven at room temperature because of the reduced mobility of water molecules in solvation shell
Solvation shell
A Solvation shell is a shell of any chemical species acting as a solvent, surrounding a solute species. When the solvent is water it is often referred to as a hydration shell or hydration sphere....
of the non-polar solute. However, the enthalpic component of transfer energy was found to be favorable, meaning strengthening of water-water hydrogen bonds in the solvation shell, apparently due to the reduced mobility of water molecules. At the higher temperature, when water molecules became more mobile, this energy gain decreases, but so does the entropic component. As a result of such entropy-enthalpy compensation, the hydrophobic effect (as measured by the free energy of transfer) is only weakly temperature-dependent and became smaller at the lower temperature, which leads to "cold denaturation
Denaturation (biochemistry)
Denaturation is a process in which proteins or nucleic acids lose their tertiary structure and secondary structure by application of some external stress or compound, such as a strong acid or base, a concentrated inorganic salt, an organic solvent , or heat...
" of proteins.
Further reading
Charles TanfordCharles Tanford
Charles Tanford was an author and one of the preeminent protein chemists of his generation. He died in York, England on October 1, 2009.-Life:Charles was born in Halle, Germany in 1921 to Majer and Charlotte Tannenbaum...
(1973). The Hydrophobic Effect: Formation of Micelles and Biological Membranes. New York, NY: John Wiley & Sons Inc. ISBN 9780471844600.

