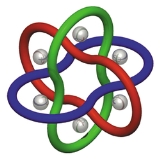
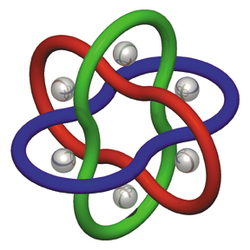
Molecular Borromean rings
Encyclopedia
Molecular Borromean rings are an example of a mechanically-interlocked molecular architecture in which three macrocycle
s are interlocked in such a way that breaking any macrocycle allows the others to disassociate. They are the smallest examples of Borromean rings
. The synthesis of molecular Borromean rings was reported in 2004 by the group of J. Fraser Stoddart
. The so-called Borromeate is made up of three interpenetrated macrocycle
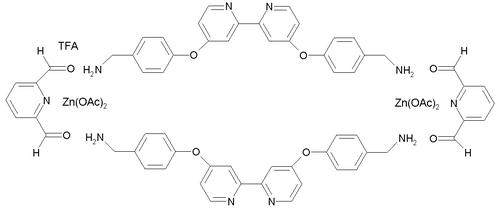
s formed from the reaction between 2,6-diformylpyridine and diamine
compounds, complexed
with zinc
.
This compound was synthesized
from two building blocks: 2,6-diformylpyridine (a pyridine
with two aldehyde
groups) and a diamine containing a 2,2'-bipyridine
group. Zinc acetate
is added as the template for the reaction, resulting in one zinc atom in each of a total of 6 pentacoordinate complexation sites. Trifluoroacetic acid
(TFA) is added to catalyse the imine
bond-forming reactions. The preparation of the tri-ring Borromeate involves a total of 18 precursor molecules and is only possible because the building blocks self-assemble
through 12 aromatic pi-pi interactions and 30 zinc to nitrogen dative bonds. Because of these interactions, the Borromeate is thermodynamically the most stable reaction product out of potentially many others. As a consequence of all the reactions taking place being equilibria
, the Borromeate is the predominant reaction product.
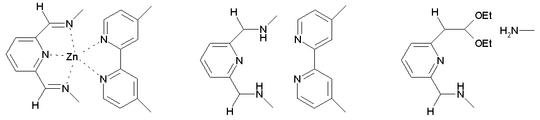
 Reduction with sodium borohydride
Reduction with sodium borohydride
in ethanol
affords the neutral Borromeand . True to a Borromean system, cleavage of just one imine
bond (to an amine
and an orthoester
) in this structure breaks the mechanical bond
between the three constituent macrocycles, releasing the other two individual rings.
 Organic synthesis of this seemingly complex compound is in reality fairly simple; the reason why the Stoddart group has suggested it for a classroom experiment on a gram scale .
Organic synthesis of this seemingly complex compound is in reality fairly simple; the reason why the Stoddart group has suggested it for a classroom experiment on a gram scale .
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
s are interlocked in such a way that breaking any macrocycle allows the others to disassociate. They are the smallest examples of Borromean rings
Borromean rings
In mathematics, the Borromean rings consist of three topological circles which are linked and form a Brunnian link, i.e., removing any ring results in two unlinked rings.- Mathematical properties :...
. The synthesis of molecular Borromean rings was reported in 2004 by the group of J. Fraser Stoddart
James Fraser Stoddart
Sir James Fraser Stoddart is a Scottish chemist currently at the Department of Chemistry at Northwestern University. He works in the area of supramolecular chemistry and nanotechnology...
. The so-called Borromeate is made up of three interpenetrated macrocycle
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
s formed from the reaction between 2,6-diformylpyridine and diamine
Diamine
A diamine is a type of polyamine with exactly two amino groups. Diamines are mainly used as monomers to prepare polyamides, polyimides and polyureas. In terms of quantities produced, 1,6-diaminohexane, a precursor to Nylon 6-6, is most important, followed by ethylenediamine...
compounds, complexed
Complex (chemistry)
In chemistry, a coordination complex or metal complex, is an atom or ion , bonded to a surrounding array of molecules or anions, that are in turn known as ligands or complexing agents...
with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
.
 |
This compound was synthesized
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
from two building blocks: 2,6-diformylpyridine (a pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
with two aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
groups) and a diamine containing a 2,2'-bipyridine
2,2'-Bipyridine
2,2'-Bipyridine is a organic compound with the formula . This colorless solid, commonly abbreviated bipy or bpy , is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals...
group. Zinc acetate
Zinc acetate
Zinc acetate is the chemical compound with the formula Zn2, which commonly occurs as a dihydrate Zn22. Both the hydrate and the anhydrous forms are colorless solids that are commonly used in chemical synthesis and as dietary supplements. Zinc acetates are prepared by the action of acetic acid on...
is added as the template for the reaction, resulting in one zinc atom in each of a total of 6 pentacoordinate complexation sites. Trifluoroacetic acid
Trifluoroacetic acid
Trifluoroacetic acid is the simplest stable perfluorinated carboxylic acid chemical compound, with the formula CF3CO2H. It is a strong carboxylic acid due to the influence of the electronegative trifluoromethyl group. TFA is almost 100,000-fold more acidic than acetic acid...
(TFA) is added to catalyse the imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
bond-forming reactions. The preparation of the tri-ring Borromeate involves a total of 18 precursor molecules and is only possible because the building blocks self-assemble
Molecular self-assembly
Molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly, intramolecular self-assembly and intermolecular self-assembly...
through 12 aromatic pi-pi interactions and 30 zinc to nitrogen dative bonds. Because of these interactions, the Borromeate is thermodynamically the most stable reaction product out of potentially many others. As a consequence of all the reactions taking place being equilibria
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, the Borromeate is the predominant reaction product.

Sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate, is an inorganic compound with the formula NaBH4. This white solid, usually encountered as a powder, is a versatile reducing agent that finds wide application in chemistry, both in the laboratory and on a technical scale. Large amounts are...
in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
affords the neutral Borromeand . True to a Borromean system, cleavage of just one imine
Imine
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond, with the nitrogen attached to a hydrogen atom or an organic group. If this group is not a hydrogen atom, then the compound is known as a Schiff base...
bond (to an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
and an orthoester
Orthoester
In organic chemistry, an orthoester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula RC3. The name can also refer to any organic compound that contains this functional group. An example of an orthoester is ethyl orthoacetate, CH3C3,...
) in this structure breaks the mechanical bond
Mechanical bond
The mechanical bond is a type of chemical bond found in mechanically-interlocked molecular architectures such as catenanes and rotaxanes. Unlike classical molecular structures, interlocked molecules consist of two or more separate components which are not connected by chemical bonds...
between the three constituent macrocycles, releasing the other two individual rings.

External links
- Three rings in an inseparable union Michel Freemantle Chemical & Engineering NewsChemical & Engineering NewsChemical & Engineering News is a weekly magazine published by the American Chemical Society, providing professional and technical information in the fields of chemistry and chemical engineering...
May 31, 2004 Volume 82, Number 22 p. 5 Article - Borromean chemistry overview website

