
Nickel aluminide
Encyclopedia
Nickel aluminide is an intermetallic material with properties similar to both a ceramic and a metal.
There are three materials called nickel aluminide:
Also known as IC-221M, this alloy
is made up of nickel
combined with several other metals including aluminium
, chromium
, molybdenum
, zirconium
and boron
. Adding boron increases the ductility
of the alloy. As well, it increases the hardness.
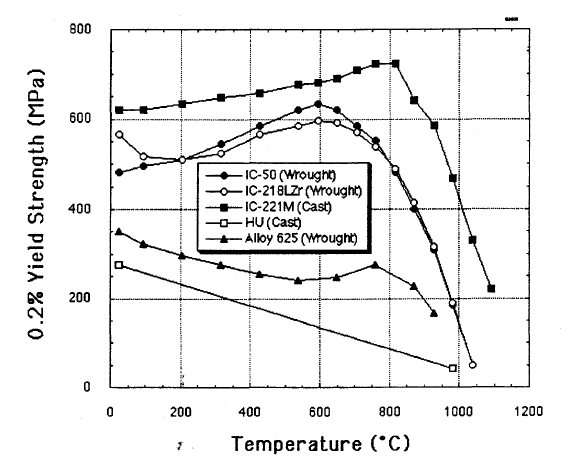
This alloy is extremely strong for its weight, five times stronger than stainless steel. Unlike most alloys, IC-221M increases in strength from room temperature up to 800 °C.
The alloy is very resistant to heat and corrosion
, and finds use in heat-treating furnace
s and other applications where its longer lifespan and reduced corrosion
give it an advantage over stainless steel
.
Nickel aluminide is unique in that it has very high thermal conductivity combined with high strength at high temperature. These properties, combined with its high strength and low density, make it ideal for special applications like coating blades in gas turbine
s and jet engine
s.
In 2005, the most abrasion-resistant material was reportedly created by embedding diamond
s in a matrix of nickel aluminide.
There are three materials called nickel aluminide:
- NiAl, CAS number 12003-78-0 (see also Raney nickelRaney nickelRaney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
) - NiAl3, CAS number 12004-71-6
- Ni3Al
Ni3Al
An intermetallic compound can be defined as an ordered alloy phase formed between two metallic elements, where an alloy phase is ordered if two or more sublattices are required to describe its atomic structure. The ordered structure exhibits superior elevated-temperature properties because of the long-range ordered superlatticeSuperlatticeSuperlattice is a periodic structure of layers of two materials. Typically, the thickness of one layer is several nanometers.- Discovery :Superlattices were discovered early in the 20th century through their special X-ray diffraction patterns....
, which reduces dislocationDislocationIn materials science, a dislocation is a crystallographic defect, or irregularity, within a crystal structure. The presence of dislocations strongly influences many of the properties of materials...
mobility and diffusionDiffusionMolecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
processes at elevated temperatures.
Also known as IC-221M, this alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
is made up of nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
combined with several other metals including aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
, chromium
Chromium
Chromium is a chemical element which has the symbol Cr and atomic number 24. It is the first element in Group 6. It is a steely-gray, lustrous, hard metal that takes a high polish and has a high melting point. It is also odorless, tasteless, and malleable...
, molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
, zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
and boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
. Adding boron increases the ductility
Ductility
In materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
of the alloy. As well, it increases the hardness.
This alloy is extremely strong for its weight, five times stronger than stainless steel. Unlike most alloys, IC-221M increases in strength from room temperature up to 800 °C.

The alloy is very resistant to heat and corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
, and finds use in heat-treating furnace
Furnace
A furnace is a device used for heating. The name derives from Latin fornax, oven.In American English and Canadian English, the term furnace on its own is generally used to describe household heating systems based on a central furnace , and sometimes as a synonym for kiln, a device used in the...
s and other applications where its longer lifespan and reduced corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
give it an advantage over stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
.
Nickel aluminide is unique in that it has very high thermal conductivity combined with high strength at high temperature. These properties, combined with its high strength and low density, make it ideal for special applications like coating blades in gas turbine
Gas turbine
A gas turbine, also called a combustion turbine, is a type of internal combustion engine. It has an upstream rotating compressor coupled to a downstream turbine, and a combustion chamber in-between....
s and jet engine
Jet engine
A jet engine is a reaction engine that discharges a fast moving jet to generate thrust by jet propulsion and in accordance with Newton's laws of motion. This broad definition of jet engines includes turbojets, turbofans, rockets, ramjets, pulse jets...
s.
In 2005, the most abrasion-resistant material was reportedly created by embedding diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
s in a matrix of nickel aluminide.
Properties
- Density = 7.26 g/cm3
- Yield Strength = 855 MPa
- Hardness = HRCRockwell scaleThe Rockwell scale is a hardness scale based on the indentation hardness of a material. The Rockwell test determines the hardness by measuring the depth of penetration of an indenter under a large load compared to the penetration made by a preload. There are different scales, denoted by a single...
12

