
Boron
Encyclopedia
Boron is the chemical element
with atomic number
5 and the chemical symbol B. Boron is a metalloid
. Because boron is not produced by stellar nucleosynthesis
, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate mineral
s. These are mined industrially as evaporate ore
s, such as borax
and kernite
.
Chemically uncombined boron is not found naturally on Earth. Industrially, very pure boron is produced with difficulty, as boron tends to form refractory materials containing small amounts of carbon or other elements. Several allotropes
of boron exist: amorphous boron is a brown powder and crystalline boron is black, extremely hard (about 9.5 on Mohs' scale), and a poor conductor at room temperature. Elemental boron is used as a dopant
in the semiconductor industry
.
The major industrial-scale uses of boron compounds are in sodium perborate
bleaches, and the borax component of fiberglass insulation. Boron polymers and ceramics play specialized roles as high-strength lightweight structural and refractory materials. Boron compounds are used in silica-based glasses and ceramics to give them resistance to thermal shock
. Boron-containing reagents are used for the synthesis of organic compounds
, as intermediate in the synthesis of fine chemicals. A few boron-containing organic pharmaceuticals are used, or are in study. Natural boron is composed of two stable isotopes, one of which (boron-10) has a number of uses as a neutron-capturing agent.
In biology, borates have low toxicity in mammals (similar to table salt), but are more toxic to arthropod
s and are used as insecticides. Boric acid is mildly antimicrobial, and a natural boron-containing organic antibiotic is known. Boron is essential to life. Small amounts of boron compounds play a strengthening role in the cell walls of all plants, making boron necessary in soils. Experiments indicate a role for boron as an ultratrace element
in animals, but the nature of its role in animal physiology is unknown.
word بورق buraq or the Persian
word بوره burah; which are names for the mineral borax
.
 Boron compounds were known thousands of years ago. Borax was known from the deserts of western Tibet, where it received the name of tincal, derived from the Sanskrit
Boron compounds were known thousands of years ago. Borax was known from the deserts of western Tibet, where it received the name of tincal, derived from the Sanskrit
. Borax glazes were used in China from AD300, and some tincal even reached the West, where the Persian alchemist Jābir ibn Hayyān seems to mention it in 700. Marco Polo
brought some glazes back to Italy in the 13th century. Agricola, around 1600, reports the use of borax as a flux in metallurgy
. In 1777, boric acid
was recognized in the hot springs (soffioni
) near Florence
, Italy, and became known as sal sedativum, with mainly medical uses. The rare mineral is called sassolite
, which is found at Sasso, Italy. Sasso was the main source of European borax from 1827 to 1872, at which date American sources replaced it. Boron compounds were relatively rarely used chemicals until the late 1800s when Francis Marion Smith
's Pacific Coast Borax Company
first popularized these compounds and made them in volume and hence cheap
Boron was not recognized as an element until it was isolated by Sir Humphry Davy
and by Joseph Louis Gay-Lussac
and Louis Jacques Thénard
In 1808 Davy observed that electric current sent through a solution of borates produced a brown precipitate on one of the electrodes. In his subsequent experiments he used potassium to reduce boric acid instead of electrolysis
. He produced enough boron to confirm a new element and named the element boracium. Gay-Lussac and Thénard use iron to reduce boric acid at high temperatures. They showed by oxidizing boron with air that boric acid is an oxidation product of boron.
Jöns Jakob Berzelius
identified boron as an element in 1824. Pure boron was arguably first produced by the American chemist Ezekiel Weintraub in 1909.
 Boron is similar to carbon
Boron is similar to carbon
in its capability to form stable covalently bonded
molecular networks. Even nominally disordered (amorphous) boron contains regular boron icosahedra which are, however, bonded randomly to each other without long-range order. Crystalline boron is a very hard, black material with a high melting point of above 2000 °C. It exists in four major polymorphs
: α, β, γ and T. Whereas α, β and T phases are based on B12 icosahedra, the γ-phase can be described as a rocksalt
-type arrangement of the icosahedra and B2 atomic pairs. It can be produced by compressing other boron phases to 12–20 GPa and heating to 1500–1800 °C; it remains stable after releasing the temperature and pressure. The T phase is produced at similar pressures, but higher temperatures of 1800–2200 °C. As to the α and β phases, they might both coexist at ambient conditions
with the β phase being more stable. Compressing boron above 160 GPa produces a boron phase with an as yet unknown structure, and this phase is a superconductor at temperatures 6–12 K.
than to aluminium
. Crystalline boron is chemically inert and resistant to attack by boiling hydrofluoric
or hydrochloric acid
. When finely divided, it is attacked slowly by hot concentrated hydrogen peroxide
, hot concentrated nitric acid
, hot sulfuric acid
or hot mixture of sulfuric and chromic acid
s.
The rate of oxidation of boron depends upon the crystallinity, particle size, purity and temperature. Boron does not react with air at room temperature, but at higher temperatures it burns to form boron trioxide
:
 Boron undergoes halogenation to give trihalides, for example:
Boron undergoes halogenation to give trihalides, for example:
These trihalides in practice are usually made from the oxides.
 In its most familiar compounds, boron has the formal oxidation state III. These include oxides, sulfides, nitrides, and halides.
In its most familiar compounds, boron has the formal oxidation state III. These include oxides, sulfides, nitrides, and halides.
The trihalides adopt a planar trigonal structure. These compounds are Lewis acid
s in that they readily form adduct
s with electron-pair donors, which are called Lewis bases. For example, fluoride (F-) and boron trifluoride
(BF3) combined to give the tetrafluoroborate
anion, BF4-. Boron trifluoride is used in the petrochemical industry as a catalyst. The halides react with water to form boric acid
.
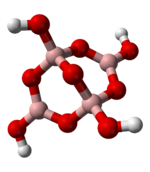
Boron is found in nature on Earth entirely as various oxides of B(III), often associated with other elements. The more than one hundred borate
s all feature boron in oxidation state +3. These mineral resemble silicates in some respect, although boron is often found not only in a tetrahedral coordination with oxygen, but also in a trigonal planar configuration. Unlike silicates, the boron minerals never feature boron with coordination number greater than four. A typical motif is exemplified by the tetraborate anions of the common mineral borax
, shown at left. The formal negative charge of the tetrahedral borate centers is balanced by metal cations in the minerals, such as the sodium (Na+) in borax.
The boron nitride
s are notable for the variety of structures that they adopt. They adopt structures analogous to various allotropes of carbon
, including graphite, diamond, and nanotubes. In the diamond-like structure called cubic boron nitride (tradename Borazon
), boron atoms exist in the tetrahedral structure of carbons atoms in diamond, but one in every four B-N bonds can be viewed as a coordinate covalent bond
, wherein two electrons are donated by the nitrogen atom which acts as the Lewis base to a bond to the Lewis acid
ic boron(III) centre. Cubic boron nitride, among other applications, is used as an abrasive, as it has a hardness comparable with diamond (the two substances are able to produce scratches on each other). In the BN compound analogue of graphite, hexagonal boron nitride (h-BN), the positively-charged boron and negatively-charged nitrogen atoms in each plane lie adjacent to the oppositely charged atom in the next plane. Consequently graphite and h-BN have very different properties: both are lubricants, as these planes slip past each other. However, h-BN is a relatively poor electrical and thermal conductor in the planar direction.
A large number of organoboron compounds are known and many are useful in organic synthesis
. Organoboron(III) compounds are usually tetrahedral or trigonal planar, for example, tetraphenylborate
(B(C6H5)4-) vs triphenylborane
(B(C6H5)3). Many are produced from hydroboration, which employs diborane
(B2H6).
Although these are not found on Earth naturally, boron forms a variety of stable compounds with formal oxidation state less than three. As for many covalent compounds, formal oxidation states are often of little meaning in boron hydrides and metal borides. The halides also form derivatives of B(I) and B(II). BF, isoelectronic with N2, is not isolable in condensed form, but B2F4
and B4Cl4 are well characterized.
 Binary metal-boron compounds, the metal borides, feature boron in oxidation state less than III. Illustrative is magnesium diboride
Binary metal-boron compounds, the metal borides, feature boron in oxidation state less than III. Illustrative is magnesium diboride
(MgB2). Each boron has a formal −1 charge and magnesium is assigned a formal charge of 2+.
In this material, the boron centers are trigonal planar, with an extra double bond for each boron, with the boron atoms forming sheets akin to the carbon in graphite
. However, unlike the case with hexagonal boron nitride which by comparison lacks electrons in the plane of the covalent atoms, the delocalized electrons in the plane of magnesium diboride allow it to conduct electricity similar to isoelectronic graphite. In addition, in 2001 this material was found to be a high-temperature superconductor.
Certain other metal borides find specialized applications as hard materials for cutting tools.
From the structural perspective, the most distinctive chemical compounds of boron are the hydrides. Included in this series are the cluster compounds dodecaborate (B12H122-), decaborane
(B10H14), and the carborane
s such as C2B10H12. Characteristically such compounds feature boron with coordination numbers greater than four.
s, 11B (80.1%) and 10B (19.9%). The mass difference results in a wide range of δ11B values, which are defined as a fractional difference between the 11B and 10B and traditionally expressed in parts per thousand, in natural waters ranging from −16 to +59. There are 13 known isotopes of boron, the shortest-lived isotope is 7B which decays through proton emission
and alpha decay
. It has a half-life
of 3.5×10−22 s
. Isotopic fractionation of boron is controlled by the exchange reactions of the boron species B(O
H
)3 and [B(OH)4]−
. Boron isotopes are also fractionated during mineral crystallization, during H2O phase changes in hydrothermal systems, and during hydrothermal alteration of rock
. The latter effect results in preferential removal of the 10B(OH)4 ion
onto clays. It results in solutions enriched in 11B(OH)3 and therefore may be responsible for the large 11B enrichment in seawater relative to both ocean
ic crust and continent
al crust; this difference may act as an isotopic signature
. The exotic 17B exhibits a nuclear halo
, i.e. its radius is appreciably larger than that predicted by the liquid drop model
.
The 10B isotope is good at capturing thermal neutrons. Natural boron is about 20% 10B and 80% 11B. The nuclear industry
enriches natural boron to nearly pure 10B. The less-valuable by-product, depleted boron, is nearly pure 11B.
adduct of boron trifluoride
(DME-BF3) and column chromatography of borates are being used.
 Enriched boron or 10B is used in both radiation shielding and in boron neutron capture therapy
Enriched boron or 10B is used in both radiation shielding and in boron neutron capture therapy
. In the latter, a compound containing 10B is attached to a muscle
near a tumor
. The patient is then treated with a relatively low dose of thermal neutrons. This causes energetic and short range alpha radiation from the boron to bombard the tumor.
In nuclear reactors, 10B is used for reactivity control and in emergency shutdown systems
. It can serve either function in the form of borosilicate control rods or as boric acid
. In pressurized water reactor
s, boric acid is added to the reactor coolant when the plant is shut down for refueling. It is then slowly filtered out over many months as fissile material is used up and the fuel becomes less reactive.
In future manned interplanetary spacecraft, 10B has a theoretical role as structural material (as boron fibers or BN nanotube
material) which would also serve a special role in the radiation shield. One of the difficulties in dealing with cosmic rays, which are mostly high energy protons, is that some secondary radiation from interaction of cosmic rays and spacecraft materials is high energy spallation
neutrons. Such neutrons can be moderated by materials high in light elements such as polyethylene
, but the moderated neutrons continue to be a radiation hazard unless actively absorbed in the shielding. Among light elements that absorb thermal neutrons, 6Li and 10B appear as potential spacecraft structural materials which serve both for mechanical reinforcement and radiation protection.
, an alpha particle
, and a lithium
ion. These resultant decay products may then irradiate nearby semiconductor 'chip' structures, causing data loss (bit flipping, or single event upset
). In radiation hardened semiconductor designs, one countermeasure is to use depleted boron which is greatly enriched in 11B and contains almost no 10B. 11B is largely immune to radiation damage. Depleted boron is a by-product of the nuclear industry
.
11B is also a candidate as a fuel for aneutronic fusion
. When struck by a proton with energy of about 500 keV
, it produces three alpha particles and 8.7 MeV of energy. Most other fusion reactions involving hydrogen and helium produce penetrating neutron radiation, which weakens reactor structures and induces long term radioactivity thereby endangering operating personnel. Whereas, the alpha particles from 11B fusion can be turned directly into electric power, and all radiation stops as soon as the reactor is turned off.
spectroscopy; and spectrometers specially adapted to detecting the boron-11 nuclei are available commercially. The 10B and 11B nuclei also cause splitting in the resonances of attached nuclei.

 Boron is a relatively rare element in the Earth's crust, representing only 0.001%. The worldwide commercial borate deposits are estimated at 10 million tonnes.
Boron is a relatively rare element in the Earth's crust, representing only 0.001%. The worldwide commercial borate deposits are estimated at 10 million tonnes.
Turkey
and the United States
are the world's largest producers of boron. Turkey has almost 72% of the world’s boron reserves. Boron does not appear on Earth in elemental form but is found combined in borax
, boric acid
, colemanite
, kernite
, ulexite
and borate
s. Boric acid is sometimes found in volcanic
spring waters.
Ulexite
is one of over a hundred borate mineral
s; it is a fibrous crystal where individual fibers can guide light like optical fibers.
Economically important sources of boron are rasorite (kernite
) and tincal (borax ore). They are both found in the Mojave Desert
of California
where the Rio Tinto Borax Mine
(also known as the U.S. Borax Boron Mine) near Boron, CA is California
's largest open-pit mine and the largest borax
mine in the world, producing nearly half the world's borates from this single site.. However, the largest borax deposits known, many still untapped, are in Central and Western Turkey
including the provinces of Eskişehir
, Kütahya
and Balıkesir
.
The earliest routes to elemental boron involved reduction of boric oxide with metals such as magnesium
or aluminium
. However the product is almost always contaminated with metal boride
s. Pure boron can be prepared by reducing volatile boron halides with hydrogen
at high temperatures. Ultrapure boron, for the use in semiconductor industry, is produced by the decomposition of diborane
at high temperatures and then further purified with the zone melting
or Czochralski process
es.
The form in which boron is consumed has changed in recent years. The use of ores like colemanite
has declined following concerns over arsenic
content. Consumers have moved towards the use of refined borates and boric acid that have a lower pollutant content. The average cost of crystalline boron is $5/g.
Increasing demand for boric acid has led a number of producers to invest in additional capacity. Eti Mine Company of Turkey opened a new boric acid plant with the production capacity of 100,000 tonnes per year at Emet
in 2003. Rio Tinto Group
increased the capacity of its boron plant from 260,000 tonnes per year in 2003 to 310,000 tonnes per year by May 2005, with plans to grow this to 366,000 tonnes per year in 2006. Chinese boron producers have been unable to meet rapidly growing demand for high quality borates. This has led to imports of sodium tetraborate (borax
) growing by a hundredfold between 2000 and 2005 and boric acid imports increasing by 28% per year over the same period.
The rise in global demand has been driven by high growth rates in fiberglass and borosilicate production. A rapid increase in the manufacture of reinforcement-grade fiberglass in Asia with a consequent increase in demand for borates has offset the development of boron-free reinforcement-grade fiberglass in Europe and the USA. The recent rises in energy prices may lead to greater use of insulation-grade fiberglass, with consequent growth in the boron consumption. Roskill Consulting Group forecasts that world demand for boron will grow by 3.4% per year to reach 21 million tonnes by 2010. The highest growth in demand is expected to be in Asia where demand could rise by an average 5.7% per year.
and sodium tetraborate pentahydrate. In the United States, 70% of the boron is used for the production of glass and ceramics.
 Borosilicate glass, which is typically 12–15% B2O3, 80% SiO2, and 2% Al2O3, has a low coefficient of thermal expansion giving it a good resistance to thermal shock. Duran and Pyrex
Borosilicate glass, which is typically 12–15% B2O3, 80% SiO2, and 2% Al2O3, has a low coefficient of thermal expansion giving it a good resistance to thermal shock. Duran and Pyrex
are two major brand names for this glass, used both in laboratory glassware
and in consumer cookware and bakeware
, chiefly for this resistance.
Boron filaments are high-strength, lightweight materials that are used chiefly for advanced aerospace
structures as a component of composite material
s, as well as limited production consumer and sporting goods such as golf club
s and fishing rod
s. The fibers can be produced by chemical vapor deposition
of boron on a tungsten
filament.
Boron fibers and sub-millimeter sized crystalline boron springs are produced by laser
-assisted chemical vapor deposition
. Translation of the focused laser beam allows to produce even complex helical structures. Such structures show good mechanical properties (elastic modulus
450 GPa, fracture strain 3.7%, fracture stress 17 GPa) and can be applied as reinforcement of ceramics or in micromechanical systems
.
" powdered hand soap. It is also present in some tooth bleaching
formulas.
Sodium perborate
serves as a source of active oxygen in many detergent
s, laundry detergent
s, cleaning products, and laundry bleaches. However, despite its name, "Borateem" laundry bleach no longer contains any boron compounds, using sodium percarbonate
instead as a bleaching agent.
for such semiconductors as silicon
, germanium
, and silicon carbide
. Having one fewer valence electron than the host atom, it donates a hole
resulting in p-type
conductivity. Traditional method of introducing boron into semiconductors is via its atomic diffusion
at high temperatures. This process uses either solid (B2O3), liquid (BBr3), or gaseous boron sources (B2H6 or BF3). However, after 1970s, it was mostly replaced by ion implantation
, which relies mostly on BF3 as a boron source. Boron trichloride gas is also an important chemical in semiconductor industry, however not for doping but rather for plasma etching
of metals and their oxides. Triethylborane is also injected into vapor deposition
reactors as a boron source. Examples are the plasma deposition of boron-containing hard carbon films, silicon nitride-boron nitride films, and for doping
of diamond
film with boron.
s (Nd2Fe14B), which are the strongest type of permanent magnet. They are found in a variety of domestic and professional electromechanical and electronic devices, such as magnetic resonance imaging
(MRI), various motors and actuator
s, computer HDDs, CD and DVD players, mobile phones, timer switches, speakers, and so on.
 Several boron compounds are known for their extreme hardness and toughness.
Several boron compounds are known for their extreme hardness and toughness.
Boron carbide and cubic boron nitride powders are widely used as abrasives. Metal boride
s are used for coating tools through chemical vapor deposition
or physical vapor deposition
. Implantation of boron ions into metals and alloys, through ion implantation
or ion beam deposition
, results in a spectacular increase in surface resistance and microhardness. Laser alloying has also been successfully used for the same purpose. These borides are an alternative to diamond coated tools, and their (treated) surfaces have similar properties to those of the bulk boride.
is a ceramic material which is obtained by decomposing B2O3 with carbon in the electric furnace:
Boron carbide's stucture is only approximately B4C, and it shows a clear depletion of carbon from this suggested stoichiometric ratio. This is due to its very complex structure. The substance can be seen with empirical formula
B12C3 (i.e., with B12 dodecahedra being a motif), but with less carbon as the suggested C3 units are replaced with B-C chains, and there are smaller (B6) octahedra present as well. (See the article for structural analysis).
The repeating polymer plus semi-crystalline structure of boron carbide gives it great structural strength per weight. It is used in tank armor
, bulletproof vest
s, and numerous other structural applications.
Boron carbide's ability to absorb neutrons without forming long-lived radionuclide
s (especially when doped with extra boron-10) makes the material attractive as an absorbent for neutron radiation arising in nuclear power plants. Nuclear applications of boron carbide include shielding, control rods and shut-down pellets. Within control rods, boron carbide is often powdered, to increase its surface area.
s, taking advantage of its high cross-section for neutron capture.

is an important superconducting material
with the transition temperature of 39 K. MgB2 wires are produced with the powder-in-tube process and applied in superconducting magnets.
Amorphous boron is used as a melting point depressant in nickel-chromium braze alloys.
Hexagonal boron nitride
forms atomically thin layers, which have been used to enhance the electron mobility
in graphene
devices. It also forms nanotubular structures (BNNTs), which have with high strength, high chemical stability, and high thermal conductivity
, among its list of desirable properties.
, boromycin
, isolated from streptomyces
. Boron is an essential plant nutrient
, required primarily for maintaining the integrity of cell walls. Conversely, high soil concentrations of > 1.0 ppm
can cause marginal and tip necrosis in leaves as well as poor overall growth performance. Levels as low as 0.8 ppm can cause these same symptoms to appear in plants particularly sensitive to boron in the soil. Nearly all plants, even those somewhat tolerant of boron in the soil, will show at least some symptoms of boron toxicity when boron content in the soil is greater than 1.8 ppm. When this content exceeds 2.0 ppm, few plants will perform well and some may not survive. When boron levels in plant tissue exceed 200 ppm symptoms of boron toxicity are likely to appear.
As an ultratrace element
, boron is necessary for the optimal health of rats, although it is necessary in such small amounts that ultrapurified foods and dust filtration of air is necessary to induce boron deficiency, which manifest as poor coat or hair quality. Presumably, boron is necessary to other mammals. No deficiency syndrome in humans has been described. Small amounts of boron occur widely in the diet, and the amounts needed in the diet would, by analogy with rodent studies, be very small. The exact physiological role of boron in the animal kingdom is poorly understood.
Boron occurs in all foods produced from plants. Since 1989 its nutritional value has been argued. It is thought that boron plays several biochemical roles in animals, including humans.
The U.S. Department of agriculture conducted an experiment in which postmenopausal women took 3 mg of boron a day. The results showed that supplemental boron reduced excretion of calcium by 44%, and activated estrogen
and vitamin D
, suggesting a possible role in the suppression of osteoporosis
. However, whether these effects were conventionally nutritional, or medicinal, could not be determined. The U.S. National Institutes of Health
states that "Total daily boron intake in normal human diet
s ranges from 2.1–4.3 mg boron/day."
curcumin method is used. Boron has to be transferred to boric acid or borate
s and on reaction with curcumin
in acidic solution, a red colored boron-chelate complex, rosocyanine
, is formed.
A number of potential boronated pharmaceuticals using boron-10, have been prepared for use in boron neutron capture therapy
(BNCT).
Some boron compounds show promise in treating arthritis
, though none have as yet been generally approved for the purpose.
Boron is used as an intermediate in pharmaceutical synthesis, but it appeared as an active element in its first-approved organic pharamaceutical in bortezomib
, a new class of drug called proteasome inhibitors, which are active in myeloma and one form of lymphoma. The boron atom in bortezomib binds the catalytic site of the 26S proteasome
with high affinity and specificity.
, boric acid
, borates, and many organoboron compounds are non-toxic to humans and animals (approximately similar to table salt). The LD50 (dose at which there is 50% mortality) for animals is about 6 g per kg of body weight. Substances with LD50 above 2 g are considered non-toxic. The minimum lethal dose for humans has not been established, but an intake of 4 g/day was reported without incidents, and medical dosages of 20 g of boric acid for neutron capture therapy caused no problems. Fish have survived for 30 min in a saturated boric acid solution and can survive longer in strong borax solutions. Boric acid is more toxic to insects than to mammals, and is routinely used as an insecticide.
The boranes and similar gaseous compounds are quite poisonous. As usual, it is not an element that is intrinsically poisonous, but toxicity depends on structure.
The borane
s (boron hydrogen compounds) are toxic as well as highly flammable and require special care when handling. Sodium borohydride presents a fire hazard due to its reducing nature, and the liberation of hydrogen on contact with acid. Boron halides are corrosive.
Congenital endothelial dystrophy type 2
, a rare form of corneal dystrophy
, is linked to mutations in SLC4A11
gene that encodes a transporter reportedly regulating the intracellular concentration of boron.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
5 and the chemical symbol B. Boron is a metalloid
Metalloid
Metalloid is a term used in chemistry when classifying the chemical elements. On the basis of their general physical and chemical properties, each element can usually be classified as a metal or a nonmetal. However, some elements with intermediate or mixed properties can be harder to characterize...
. Because boron is not produced by stellar nucleosynthesis
Stellar nucleosynthesis
Stellar nucleosynthesis is the collective term for the nuclear reactions taking place in stars to build the nuclei of the elements heavier than hydrogen. Some small quantity of these reactions also occur on the stellar surface under various circumstances...
, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate mineral
Borate mineral
The borate minerals are minerals which contain a borate anion group. The borate units may be polymerised similar to the SiO4 unit of the silicate mineral class. This results in B2O5, B3O6, B2O4 anions as well as more complex structures which include hydroxide or halogen anions...
s. These are mined industrially as evaporate ore
Ore
An ore is a type of rock that contains minerals with important elements including metals. The ores are extracted through mining; these are then refined to extract the valuable element....
s, such as borax
Borax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.Borax has a wide variety of uses...
and kernite
Kernite
Kernite, also known as rasorite is a hydrated sodium borate hydroxide mineral with formula Na2B4O62·3H2O. It is a colorless to white mineral crystallizing in the monoclinic crystal system typically occurring as prismatic to acicular crystals or granular masses. It is relatively soft with Mohs...
.
Chemically uncombined boron is not found naturally on Earth. Industrially, very pure boron is produced with difficulty, as boron tends to form refractory materials containing small amounts of carbon or other elements. Several allotropes
Allotropy
Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, known as allotropes of these elements...
of boron exist: amorphous boron is a brown powder and crystalline boron is black, extremely hard (about 9.5 on Mohs' scale), and a poor conductor at room temperature. Elemental boron is used as a dopant
Dopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
in the semiconductor industry
Semiconductor industry
The semiconductor industry is the aggregate collection of companies engaged in the design and fabrication of semiconductor devices. It formed around 1960, once the fabrication of semiconductors became a viable business...
.
The major industrial-scale uses of boron compounds are in sodium perborate
Sodium perborate
Sodium perborate is a white, odorless, water-soluble chemical compound with the chemical composition 3. It crystallizes as the monohydrate, NaBO3·H2O, trihydrate, NaBO3·3H2O and tetrahydrate, NaBO3·4H2O. The monohydrate and tetrahydrate are the commercially important forms...
bleaches, and the borax component of fiberglass insulation. Boron polymers and ceramics play specialized roles as high-strength lightweight structural and refractory materials. Boron compounds are used in silica-based glasses and ceramics to give them resistance to thermal shock
Thermal shock
Thermal shock is the name given to cracking as a result of rapid temperature change. Glass and ceramic objects are particularly vulnerable to this form of failure, due to their low toughness, low thermal conductivity, and high thermal expansion coefficients...
. Boron-containing reagents are used for the synthesis of organic compounds
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, as intermediate in the synthesis of fine chemicals. A few boron-containing organic pharmaceuticals are used, or are in study. Natural boron is composed of two stable isotopes, one of which (boron-10) has a number of uses as a neutron-capturing agent.
In biology, borates have low toxicity in mammals (similar to table salt), but are more toxic to arthropod
Arthropod
An arthropod is an invertebrate animal having an exoskeleton , a segmented body, and jointed appendages. Arthropods are members of the phylum Arthropoda , and include the insects, arachnids, crustaceans, and others...
s and are used as insecticides. Boric acid is mildly antimicrobial, and a natural boron-containing organic antibiotic is known. Boron is essential to life. Small amounts of boron compounds play a strengthening role in the cell walls of all plants, making boron necessary in soils. Experiments indicate a role for boron as an ultratrace element
Ultratrace element
In biochemistry, an ultratrace element is a chemical element that normally comprises less than one microgram per gram of a given organism , but which plays a significant role in its metabolism....
in animals, but the nature of its role in animal physiology is unknown.
History and etymology
The name boron originates from the ArabicArabic language
Arabic is a name applied to the descendants of the Classical Arabic language of the 6th century AD, used most prominently in the Quran, the Islamic Holy Book...
word بورق buraq or the Persian
Persian language
Persian is an Iranian language within the Indo-Iranian branch of the Indo-European languages. It is primarily spoken in Iran, Afghanistan, Tajikistan and countries which historically came under Persian influence...
word بوره burah; which are names for the mineral borax
Borax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.Borax has a wide variety of uses...
.

Sanskrit
Sanskrit , is a historical Indo-Aryan language and the primary liturgical language of Hinduism, Jainism and Buddhism.Buddhism: besides Pali, see Buddhist Hybrid Sanskrit Today, it is listed as one of the 22 scheduled languages of India and is an official language of the state of Uttarakhand...
. Borax glazes were used in China from AD300, and some tincal even reached the West, where the Persian alchemist Jābir ibn Hayyān seems to mention it in 700. Marco Polo
Marco Polo
Marco Polo was a Venetian merchant traveler from the Venetian Republic whose travels are recorded in Il Milione, a book which did much to introduce Europeans to Central Asia and China. He learned about trading whilst his father and uncle, Niccolò and Maffeo, travelled through Asia and apparently...
brought some glazes back to Italy in the 13th century. Agricola, around 1600, reports the use of borax as a flux in metallurgy
Metallurgy
Metallurgy is a domain of materials science that studies the physical and chemical behavior of metallic elements, their intermetallic compounds, and their mixtures, which are called alloys. It is also the technology of metals: the way in which science is applied to their practical use...
. In 1777, boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
was recognized in the hot springs (soffioni
Soffioni
Soffioni , a name applied in Italy to certain volcanic vents which emit jets of steam, generally associated with hydrogen sulfide and carbon dioxide, sometimes also with a little ammonia and marsh gas....
) near Florence
Florence
Florence is the capital city of the Italian region of Tuscany and of the province of Florence. It is the most populous city in Tuscany, with approximately 370,000 inhabitants, expanding to over 1.5 million in the metropolitan area....
, Italy, and became known as sal sedativum, with mainly medical uses. The rare mineral is called sassolite
Sassolite
Sassolite is a borate mineral, and is the mineral form of boric acid. It occurs in volcanic fumaroles and hot springs, as well as in bedded sedimentary evaporite deposits....
, which is found at Sasso, Italy. Sasso was the main source of European borax from 1827 to 1872, at which date American sources replaced it. Boron compounds were relatively rarely used chemicals until the late 1800s when Francis Marion Smith
Francis Marion Smith
Francis Marion Smith was an American miner, business magnate and civic builder in the Mojave Desert, the San Francisco Bay Area, and Oakland, California.Frank Smith created the extensive interurban public transit Key System in Oakland, the East Bay,...
's Pacific Coast Borax Company
Pacific Coast Borax Company
The Pacific Coast Borax Company was a United States mining company founded in 1890 by the American borax magnate Francis "Borax" Smith, the "Borax King".-History:...
first popularized these compounds and made them in volume and hence cheap
Boron was not recognized as an element until it was isolated by Sir Humphry Davy
Humphry Davy
Sir Humphry Davy, 1st Baronet FRS MRIA was a British chemist and inventor. He is probably best remembered today for his discoveries of several alkali and alkaline earth metals, as well as contributions to the discoveries of the elemental nature of chlorine and iodine...
and by Joseph Louis Gay-Lussac
Joseph Louis Gay-Lussac
- External links :* from the American Chemical Society* from the Encyclopædia Britannica, 10th Edition * , Paris...
and Louis Jacques Thénard
Louis Jacques Thénard
Louis Jacques Thénard , was a French chemist.His father, a poor peasant, managed to have him educated at the academy of Sens, and sent him at the age of sixteen to study pharmacy in Paris. There he attended the lectures of Antoine François Fourcroy and Louis Nicolas Vauquelin...
In 1808 Davy observed that electric current sent through a solution of borates produced a brown precipitate on one of the electrodes. In his subsequent experiments he used potassium to reduce boric acid instead of electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
. He produced enough boron to confirm a new element and named the element boracium. Gay-Lussac and Thénard use iron to reduce boric acid at high temperatures. They showed by oxidizing boron with air that boric acid is an oxidation product of boron.
Jöns Jakob Berzelius
Jöns Jakob Berzelius
Jöns Jacob Berzelius was a Swedish chemist. He worked out the modern technique of chemical formula notation, and is together with John Dalton, Antoine Lavoisier, and Robert Boyle considered a father of modern chemistry...
identified boron as an element in 1824. Pure boron was arguably first produced by the American chemist Ezekiel Weintraub in 1909.
Allotropes

Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
in its capability to form stable covalently bonded
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
molecular networks. Even nominally disordered (amorphous) boron contains regular boron icosahedra which are, however, bonded randomly to each other without long-range order. Crystalline boron is a very hard, black material with a high melting point of above 2000 °C. It exists in four major polymorphs
Polymorphism (materials science)
Polymorphism in materials science is the ability of a solid material to exist in more than one form or crystal structure. Polymorphism can potentially be found in any crystalline material including polymers, minerals, and metals, and is related to allotropy, which refers to chemical elements...
: α, β, γ and T. Whereas α, β and T phases are based on B12 icosahedra, the γ-phase can be described as a rocksalt
Sodium chloride
Sodium chloride, also known as salt, common salt, table salt or halite, is an inorganic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms...
-type arrangement of the icosahedra and B2 atomic pairs. It can be produced by compressing other boron phases to 12–20 GPa and heating to 1500–1800 °C; it remains stable after releasing the temperature and pressure. The T phase is produced at similar pressures, but higher temperatures of 1800–2200 °C. As to the α and β phases, they might both coexist at ambient conditions
Standard conditions for temperature and pressure
Standard condition for temperature and pressure are standard sets of conditions for experimental measurements established to allow comparisons to be made between different sets of data...
with the β phase being more stable. Compressing boron above 160 GPa produces a boron phase with an as yet unknown structure, and this phase is a superconductor at temperatures 6–12 K.
| Boron phase | α | β | γ | T |
|---|---|---|---|---|
| Symmetry | Rhombohedral | Rhombohedral | Orthorhombic | Tetragonal |
| Atoms/unit cell | 12 | ~105 | 28 | |
| Density (g/cm3) | 2.46 | 2.35 | 2.52 | 2.36 |
| Vickers hardness (GPa) | 42 | 45 | 50–58 | |
| Bulk modulus Bulk modulus The bulk modulus of a substance measures the substance's resistance to uniform compression. It is defined as the pressure increase needed to decrease the volume by a factor of 1/e... (GPa) |
185 | 224 | 227 | |
| Bandgap (eV) | 2 | 1.6 | 2.1 |
Chemistry of the element
Elemental boron is rare and poorly studied because the material is extremely difficult to prepare. Most studies on "boron" involve samples that contain small amounts of carbon. Chemically, boron behaves more closely to siliconSilicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
than to aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
. Crystalline boron is chemically inert and resistant to attack by boiling hydrofluoric
Hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride in water. It is a valued source of fluorine and is the precursor to numerous pharmaceuticals such as fluoxetine and diverse materials such as PTFE ....
or hydrochloric acid
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
. When finely divided, it is attacked slowly by hot concentrated hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
, hot concentrated nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
, hot sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
or hot mixture of sulfuric and chromic acid
Chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the...
s.
The rate of oxidation of boron depends upon the crystallinity, particle size, purity and temperature. Boron does not react with air at room temperature, but at higher temperatures it burns to form boron trioxide
Boron trioxide
Boron trioxide is one of the oxides of boron. It is a white, glassy solid with the formula B2O3. It is almost always found as the vitreous form; however, it can be crystallized after extensive annealing...
:
- 4 B + 3 O2 → 2 B2O3

- 2 B + 3 Br2 → 2 BBr3
These trihalides in practice are usually made from the oxides.
Chemical compounds

The trihalides adopt a planar trigonal structure. These compounds are Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
s in that they readily form adduct
Adduct
An adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species...
s with electron-pair donors, which are called Lewis bases. For example, fluoride (F-) and boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
(BF3) combined to give the tetrafluoroborate
Tetrafluoroborate
Tetrafluoroborate is the anion BF4−. This tetrahedral species is isoelectronic with tetrafluoromethane, CF4 and tetrafluoroammonium NF4+, and is valence isoelectronic with many stable and important species including the closely related anion perchlorate, ClO4−...
anion, BF4-. Boron trifluoride is used in the petrochemical industry as a catalyst. The halides react with water to form boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
.
Boron is found in nature on Earth entirely as various oxides of B(III), often associated with other elements. The more than one hundred borate
Borate mineral
The borate minerals are minerals which contain a borate anion group. The borate units may be polymerised similar to the SiO4 unit of the silicate mineral class. This results in B2O5, B3O6, B2O4 anions as well as more complex structures which include hydroxide or halogen anions...
s all feature boron in oxidation state +3. These mineral resemble silicates in some respect, although boron is often found not only in a tetrahedral coordination with oxygen, but also in a trigonal planar configuration. Unlike silicates, the boron minerals never feature boron with coordination number greater than four. A typical motif is exemplified by the tetraborate anions of the common mineral borax
Borax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.Borax has a wide variety of uses...
, shown at left. The formal negative charge of the tetrahedral borate centers is balanced by metal cations in the minerals, such as the sodium (Na+) in borax.
The boron nitride
Boron nitride
Boron nitride is a chemical compound with chemical formula BN, consisting of equal numbers of boron and nitrogen atoms. BN is isoelectronic to a similarly structured carbon lattice and thus exists in various crystalline forms...
s are notable for the variety of structures that they adopt. They adopt structures analogous to various allotropes of carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, including graphite, diamond, and nanotubes. In the diamond-like structure called cubic boron nitride (tradename Borazon
Borazon
Borazon is a brand name of a cubic form of boron nitride . It is one of the hardest known materials, along with various forms of diamond and boron nitride. Borazon is a crystal created by heating equal quantities of boron and nitrogen at temperatures greater than 1800 °C at 7 GPa...
), boron atoms exist in the tetrahedral structure of carbons atoms in diamond, but one in every four B-N bonds can be viewed as a coordinate covalent bond
Coordinate covalent bond
A dipolar bond, also known as dative covalent bond or coordinate bond is a kind of 2-centre, 2-electron covalent bond in which the two electrons derive from the same atom. Typically, a dipolar bond is formed when a Lewis base donates a pair of electrons to a Lewis acid. This description of bonding...
, wherein two electrons are donated by the nitrogen atom which acts as the Lewis base to a bond to the Lewis acid
Lewis acid
]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
ic boron(III) centre. Cubic boron nitride, among other applications, is used as an abrasive, as it has a hardness comparable with diamond (the two substances are able to produce scratches on each other). In the BN compound analogue of graphite, hexagonal boron nitride (h-BN), the positively-charged boron and negatively-charged nitrogen atoms in each plane lie adjacent to the oppositely charged atom in the next plane. Consequently graphite and h-BN have very different properties: both are lubricants, as these planes slip past each other. However, h-BN is a relatively poor electrical and thermal conductor in the planar direction.
Organoboron chemistry
A large number of organoboron compounds are known and many are useful in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
. Organoboron(III) compounds are usually tetrahedral or trigonal planar, for example, tetraphenylborate
Tetraphenylborate
Tetraphenylborate is an organoboron anion consisting of a central boron atom with four phenyl groups. Tetraphenylborate uncouples oxidative phosphorylation....
(B(C6H5)4-) vs triphenylborane
Triphenylborane
Triphenylborane, often abbreviated to BPh3 where Ph is the phenyl group C6H5-, is a chemical compound with the formula B3. It is a white crystalline solid and is both air and moisture sensitive, slowly forming benzene and triphenylboroxine. It is soluble in aromatic solvents.-Structure and...
(B(C6H5)3). Many are produced from hydroboration, which employs diborane
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
(B2H6).
Compounds of B(I) and B(II)
Although these are not found on Earth naturally, boron forms a variety of stable compounds with formal oxidation state less than three. As for many covalent compounds, formal oxidation states are often of little meaning in boron hydrides and metal borides. The halides also form derivatives of B(I) and B(II). BF, isoelectronic with N2, is not isolable in condensed form, but B2F4
Diboron tetrafluoride
Diboron tetrafluoride is a colorless gas. It can be formed by reacting boron monofluoride with boron trifluoride at low temperatures, taking care not to form higher polymers.-External links:*...
and B4Cl4 are well characterized.

Magnesium diboride
Magnesium diboride is a simple ionic binary compound that has proven to be an inexpensive and useful superconducting material.Its superconductivity was announced in the journal Nature in March 2001. Its critical temperature of is the highest amongst conventional superconductors...
(MgB2). Each boron has a formal −1 charge and magnesium is assigned a formal charge of 2+.
In this material, the boron centers are trigonal planar, with an extra double bond for each boron, with the boron atoms forming sheets akin to the carbon in graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
. However, unlike the case with hexagonal boron nitride which by comparison lacks electrons in the plane of the covalent atoms, the delocalized electrons in the plane of magnesium diboride allow it to conduct electricity similar to isoelectronic graphite. In addition, in 2001 this material was found to be a high-temperature superconductor.
Certain other metal borides find specialized applications as hard materials for cutting tools.
From the structural perspective, the most distinctive chemical compounds of boron are the hydrides. Included in this series are the cluster compounds dodecaborate (B12H122-), decaborane
Decaborane
Decaborane, also called decaborane, is the borane with the chemical formula B10H14. This white crystalline compound is one of the principal boron hydride clusters, both as a reference structure and as a precursor to other boron hydrides....
(B10H14), and the carborane
Carborane
A carborane is a cluster composed of boron and carbon atoms. Like many of the related boranes, these clusters are polyhedra and are similarly classified as closo-, nido-, arachno-, hypho-, etc...
s such as C2B10H12. Characteristically such compounds feature boron with coordination numbers greater than four.
Isotopes
Boron has two naturally occurring and stable isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s, 11B (80.1%) and 10B (19.9%). The mass difference results in a wide range of δ11B values, which are defined as a fractional difference between the 11B and 10B and traditionally expressed in parts per thousand, in natural waters ranging from −16 to +59. There are 13 known isotopes of boron, the shortest-lived isotope is 7B which decays through proton emission
Proton emission
Proton emission is a type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state of very...
and alpha decay
Alpha decay
Alpha decay is a type of radioactive decay in which an atomic nucleus emits an alpha particle and thereby transforms into an atom with a mass number 4 less and atomic number 2 less...
. It has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 3.5×10−22 s
Second
The second is a unit of measurement of time, and is the International System of Units base unit of time. It may be measured using a clock....
. Isotopic fractionation of boron is controlled by the exchange reactions of the boron species B(O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
)3 and [B(OH)4]−
Tetrahydroxyborate
Tetrahydroxyborate, [H4BO4]− or B, is a boron oxoanion with a tetrahedral geometry. It is isoelectronic with the hypothetical compound orthocarbonic acid.B is formed by the addition of hydroxide, OH−, to boric acid, B3:...
. Boron isotopes are also fractionated during mineral crystallization, during H2O phase changes in hydrothermal systems, and during hydrothermal alteration of rock
Rock (geology)
In geology, rock or stone is a naturally occurring solid aggregate of minerals and/or mineraloids.The Earth's outer solid layer, the lithosphere, is made of rock. In general rocks are of three types, namely, igneous, sedimentary, and metamorphic...
. The latter effect results in preferential removal of the 10B(OH)4 ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
onto clays. It results in solutions enriched in 11B(OH)3 and therefore may be responsible for the large 11B enrichment in seawater relative to both ocean
Ocean
An ocean is a major body of saline water, and a principal component of the hydrosphere. Approximately 71% of the Earth's surface is covered by ocean, a continuous body of water that is customarily divided into several principal oceans and smaller seas.More than half of this area is over 3,000...
ic crust and continent
Continent
A continent is one of several very large landmasses on Earth. They are generally identified by convention rather than any strict criteria, with seven regions commonly regarded as continents—they are : Asia, Africa, North America, South America, Antarctica, Europe, and Australia.Plate tectonics is...
al crust; this difference may act as an isotopic signature
Isotopic signature
An isotopic signature is a ratio of stable or unstable isotopes of particular elements found in an investigated material...
. The exotic 17B exhibits a nuclear halo
Nuclear halo
In nuclear physics, an atomic nucleus is called a halo nucleus or is said to have a nuclear halo if its radius is appreciably larger than that predicted by the liquid drop model, wherein the nucleus is assumed to be a sphere of constant density....
, i.e. its radius is appreciably larger than that predicted by the liquid drop model
Semi-empirical mass formula
In nuclear physics, the semi-empirical mass formula is used to approximate the mass and various other properties of an atomic nucleus...
.
The 10B isotope is good at capturing thermal neutrons. Natural boron is about 20% 10B and 80% 11B. The nuclear industry
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
enriches natural boron to nearly pure 10B. The less-valuable by-product, depleted boron, is nearly pure 11B.
Commercial isotope enrichment
Because of its high neutron cross-section, boron-10 is often used to control fission in nuclear reactors as a neutron-capturing substance. Several industrial-scale enrichment processes have been developed, however only the fractionated vacuum distillation of the dimethyl etherDimethyl ether
Dimethyl ether , also known as methoxymethane, is the organic compound with the formula . The simplest ether, it is a colourless gas that is a useful precursor to other organic compounds and an aerosol propellant. When combusted, DME produces minimal soot and CO, though HC and NOx formation is...
adduct of boron trifluoride
Boron trifluoride
Boron trifluoride is the chemical compound with the formula BF3. This pungent colourless toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.-Structure and bonding:...
(DME-BF3) and column chromatography of borates are being used.
Enriched boron (boron-10)

Boron Neutron Capture Therapy
Boron neutron capture therapy is an experimental form of radiotherapy that uses a neutron beam that interacts with boron injected into a patient...
. In the latter, a compound containing 10B is attached to a muscle
Muscle
Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to...
near a tumor
Tumor
A tumor or tumour is commonly used as a synonym for a neoplasm that appears enlarged in size. Tumor is not synonymous with cancer...
. The patient is then treated with a relatively low dose of thermal neutrons. This causes energetic and short range alpha radiation from the boron to bombard the tumor.
In nuclear reactors, 10B is used for reactivity control and in emergency shutdown systems
Scram
A scram or SCRAM is an emergency shutdown of a nuclear reactor – though the term has been extended to cover shutdowns of other complex operations, such as server farms and even large model railroads...
. It can serve either function in the form of borosilicate control rods or as boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
. In pressurized water reactor
Pressurized water reactor
Pressurized water reactors constitute a large majority of all western nuclear power plants and are one of three types of light water reactor , the other types being boiling water reactors and supercritical water reactors...
s, boric acid is added to the reactor coolant when the plant is shut down for refueling. It is then slowly filtered out over many months as fissile material is used up and the fuel becomes less reactive.
In future manned interplanetary spacecraft, 10B has a theoretical role as structural material (as boron fibers or BN nanotube
Inorganic nanotube
An inorganic nanotube is a cylindrical molecule often composed of metal oxides, and morphologically similar to a carbon nanotube. Inorganic nanotubes have been observed to occur naturally in some mineral deposits....
material) which would also serve a special role in the radiation shield. One of the difficulties in dealing with cosmic rays, which are mostly high energy protons, is that some secondary radiation from interaction of cosmic rays and spacecraft materials is high energy spallation
Spallation
In general, spallation is a process in which fragments of material are ejected from a body due to impact or stress. In the context of impact mechanics it describes ejection or vaporization of material from a target during impact by a projectile...
neutrons. Such neutrons can be moderated by materials high in light elements such as polyethylene
Polyethylene
Polyethylene or polythene is the most widely used plastic, with an annual production of approximately 80 million metric tons...
, but the moderated neutrons continue to be a radiation hazard unless actively absorbed in the shielding. Among light elements that absorb thermal neutrons, 6Li and 10B appear as potential spacecraft structural materials which serve both for mechanical reinforcement and radiation protection.
Depleted boron (boron-11)
Cosmic radiation will produce secondary neutrons if it hits spacecraft structures. Those neutrons will be captured in 10B, if it is present in the spacecraft's semiconductors, producing a gamma rayGamma ray
Gamma radiation, also known as gamma rays or hyphenated as gamma-rays and denoted as γ, is electromagnetic radiation of high frequency . Gamma rays are usually naturally produced on Earth by decay of high energy states in atomic nuclei...
, an alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
, and a lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
ion. These resultant decay products may then irradiate nearby semiconductor 'chip' structures, causing data loss (bit flipping, or single event upset
Single event upset
A single event upset is a change of state caused by ions or electro-magnetic radiation striking a sensitive node in a micro-electronic device, such as in a microprocessor, semiconductor memory, or power transistors. The state change is a result of the free charge created by ionization in or close...
). In radiation hardened semiconductor designs, one countermeasure is to use depleted boron which is greatly enriched in 11B and contains almost no 10B. 11B is largely immune to radiation damage. Depleted boron is a by-product of the nuclear industry
Nuclear power
Nuclear power is the use of sustained nuclear fission to generate heat and electricity. Nuclear power plants provide about 6% of the world's energy and 13–14% of the world's electricity, with the U.S., France, and Japan together accounting for about 50% of nuclear generated electricity...
.
11B is also a candidate as a fuel for aneutronic fusion
Aneutronic fusion
Aneutronic fusion is any form of fusion power where neutrons carry no more than 1% of the total released energy. The most-studied fusion reactions release up to 80% of their energy in neutrons...
. When struck by a proton with energy of about 500 keV
Electronvolt
In physics, the electron volt is a unit of energy equal to approximately joule . By definition, it is equal to the amount of kinetic energy gained by a single unbound electron when it accelerates through an electric potential difference of one volt...
, it produces three alpha particles and 8.7 MeV of energy. Most other fusion reactions involving hydrogen and helium produce penetrating neutron radiation, which weakens reactor structures and induces long term radioactivity thereby endangering operating personnel. Whereas, the alpha particles from 11B fusion can be turned directly into electric power, and all radiation stops as soon as the reactor is turned off.
NMR spectroscopy
Both 10B and 11B possess nuclear spin. The nuclear spin of 10B is 3 and that of 11B is 3/2. These isotopes are, therefore, of use in nuclear magnetic resonanceNuclear magnetic resonance
Nuclear magnetic resonance is a physical phenomenon in which magnetic nuclei in a magnetic field absorb and re-emit electromagnetic radiation...
spectroscopy; and spectrometers specially adapted to detecting the boron-11 nuclei are available commercially. The 10B and 11B nuclei also cause splitting in the resonances of attached nuclei.
Occurrence


Turkey
Turkey
Turkey , known officially as the Republic of Turkey , is a Eurasian country located in Western Asia and in East Thrace in Southeastern Europe...
and the United States
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
are the world's largest producers of boron. Turkey has almost 72% of the world’s boron reserves. Boron does not appear on Earth in elemental form but is found combined in borax
Borax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.Borax has a wide variety of uses...
, boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
, colemanite
Colemanite
Colemanite is a borate mineral found in evaporite deposits of alkaline lacustrine environments. Colemanite is a secondary mineral that forms by alteration of borax and ulexite....
, kernite
Kernite
Kernite, also known as rasorite is a hydrated sodium borate hydroxide mineral with formula Na2B4O62·3H2O. It is a colorless to white mineral crystallizing in the monoclinic crystal system typically occurring as prismatic to acicular crystals or granular masses. It is relatively soft with Mohs...
, ulexite
Ulexite
Ulexite , sometimes known as TV rock, is a mineral occurring in silky white rounded crystalline masses or in parallel fibers. The natural fibers of ulexite conduct light along their long axes, by internal reflection...
and borate
Borate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
s. Boric acid is sometimes found in volcanic
Volcano
2. Bedrock3. Conduit 4. Base5. Sill6. Dike7. Layers of ash emitted by the volcano8. Flank| 9. Layers of lava emitted by the volcano10. Throat11. Parasitic cone12. Lava flow13. Vent14. Crater15...
spring waters.
Ulexite
Ulexite
Ulexite , sometimes known as TV rock, is a mineral occurring in silky white rounded crystalline masses or in parallel fibers. The natural fibers of ulexite conduct light along their long axes, by internal reflection...
is one of over a hundred borate mineral
Borate mineral
The borate minerals are minerals which contain a borate anion group. The borate units may be polymerised similar to the SiO4 unit of the silicate mineral class. This results in B2O5, B3O6, B2O4 anions as well as more complex structures which include hydroxide or halogen anions...
s; it is a fibrous crystal where individual fibers can guide light like optical fibers.
Economically important sources of boron are rasorite (kernite
Kernite
Kernite, also known as rasorite is a hydrated sodium borate hydroxide mineral with formula Na2B4O62·3H2O. It is a colorless to white mineral crystallizing in the monoclinic crystal system typically occurring as prismatic to acicular crystals or granular masses. It is relatively soft with Mohs...
) and tincal (borax ore). They are both found in the Mojave Desert
Mojave Desert
The Mojave Desert occupies a significant portion of southeastern California and smaller parts of central California, southern Nevada, southwestern Utah and northwestern Arizona, in the United States...
of California
California
California is a state located on the West Coast of the United States. It is by far the most populous U.S. state, and the third-largest by land area...
where the Rio Tinto Borax Mine
Rio Tinto Borax Mine
The Rio Tinto Borax Mine in Boron, CA is California's largest open-pit mine and the largest borax mine in the world, producing nearly half the world's borates. It is operated by the Rio Tinto Group....
(also known as the U.S. Borax Boron Mine) near Boron, CA is California
California
California is a state located on the West Coast of the United States. It is by far the most populous U.S. state, and the third-largest by land area...
's largest open-pit mine and the largest borax
Borax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.Borax has a wide variety of uses...
mine in the world, producing nearly half the world's borates from this single site.. However, the largest borax deposits known, many still untapped, are in Central and Western Turkey
Turkey
Turkey , known officially as the Republic of Turkey , is a Eurasian country located in Western Asia and in East Thrace in Southeastern Europe...
including the provinces of Eskişehir
Eskisehir
Eskişehir is a city in northwestern Turkey and the capital of the Eskişehir Province. According to the 2009 census, the population of the city is 631,905. The city is located on the banks of the Porsuk River, 792 m above sea level, where it overlooks the fertile Phrygian Valley. In the nearby...
, Kütahya
Kütahya
Kütahya is a city in western Turkey with 212,444 inhabitants , lying on the Porsuk river, at 969 metres above sea level. It is the capital of Kütahya Province, inhabited by some 517 804 people...
and Balıkesir
Balikesir
Balıkesir is the capital city of Balıkesir Province. Balıkesir is in the Marmara region of Turkey and has a population of 265,747 inhabitants. Old name is Karesi or Karasi.- History :...
.
Production
The production of boron compounds does not involve formation of elemental boron, but exploits the convenient availability of borates.The earliest routes to elemental boron involved reduction of boric oxide with metals such as magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
or aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
. However the product is almost always contaminated with metal boride
Boride
In chemistry a boride is a chemical compound between boron and a less electronegative element, for example silicon boride . The borides are a very large group of compounds that are generally high melting and are not ionic in nature. Some borides exhibit very useful physical properties. The term...
s. Pure boron can be prepared by reducing volatile boron halides with hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
at high temperatures. Ultrapure boron, for the use in semiconductor industry, is produced by the decomposition of diborane
Diborane
Diborane is the chemical compound consisting of boron and hydrogen with the formula B2H6. It is a colorless gas at room temperature with a repulsively sweet odor. Diborane mixes well with air, easily forming explosive mixtures. Diborane will ignite spontaneously in moist air at room temperature...
at high temperatures and then further purified with the zone melting
Zone melting
Zone melting is a group of similar methods of purifying crystals, in which a narrow region of a crystal is molten, and this molten zone is moved along the crystal...
or Czochralski process
Czochralski process
The Czochralski process is a method of crystal growth used to obtain single crystals of semiconductors , metals , salts, and synthetic gemstones...
es.
Market trend
Estimated global consumption of boron rose to a record 1.8 million tonnes of B2O3 in 2005, following a period of strong growth in demand from Asia, Europe and North America. Boron mining and refining capacities are considered to be adequate to meet expected levels of growth through the next decade.The form in which boron is consumed has changed in recent years. The use of ores like colemanite
Colemanite
Colemanite is a borate mineral found in evaporite deposits of alkaline lacustrine environments. Colemanite is a secondary mineral that forms by alteration of borax and ulexite....
has declined following concerns over arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
content. Consumers have moved towards the use of refined borates and boric acid that have a lower pollutant content. The average cost of crystalline boron is $5/g.
Increasing demand for boric acid has led a number of producers to invest in additional capacity. Eti Mine Company of Turkey opened a new boric acid plant with the production capacity of 100,000 tonnes per year at Emet
Emet
Emet is a town and a district of Kütahya Province in the Aegean region of Turkey.It is also a Hebrew word meaning "truth", often used in the legend of the Golem. This word was written across the forehead of the mythical beast by the operator...
in 2003. Rio Tinto Group
Rio Tinto Group
The Rio Tinto Group is a diversified, British-Australian, multinational mining and resources group with headquarters in London and Melbourne. The company was founded in 1873, when a multinational consortium of investors purchased a mine complex on the Rio Tinto river, in Huelva, Spain from the...
increased the capacity of its boron plant from 260,000 tonnes per year in 2003 to 310,000 tonnes per year by May 2005, with plans to grow this to 366,000 tonnes per year in 2006. Chinese boron producers have been unable to meet rapidly growing demand for high quality borates. This has led to imports of sodium tetraborate (borax
Borax
Borax, also known as sodium borate, sodium tetraborate, or disodium tetraborate, is an important boron compound, a mineral, and a salt of boric acid. It is usually a white powder consisting of soft colorless crystals that dissolve easily in water.Borax has a wide variety of uses...
) growing by a hundredfold between 2000 and 2005 and boric acid imports increasing by 28% per year over the same period.
The rise in global demand has been driven by high growth rates in fiberglass and borosilicate production. A rapid increase in the manufacture of reinforcement-grade fiberglass in Asia with a consequent increase in demand for borates has offset the development of boron-free reinforcement-grade fiberglass in Europe and the USA. The recent rises in energy prices may lead to greater use of insulation-grade fiberglass, with consequent growth in the boron consumption. Roskill Consulting Group forecasts that world demand for boron will grow by 3.4% per year to reach 21 million tonnes by 2010. The highest growth in demand is expected to be in Asia where demand could rise by an average 5.7% per year.
Applications
Nearly all boron ore extracted from the Earth is destined for refinement into boric acidBoric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
and sodium tetraborate pentahydrate. In the United States, 70% of the boron is used for the production of glass and ceramics.
Glass and ceramics

Pyrex
Pyrex is a brand name for glassware, introduced by Corning Incorporated in 1915.Originally, Pyrex was made from borosilicate glass. In the 1940s the composition was changed for some products to tempered soda-lime glass, which is the most common form of glass used in glass bakeware in the US and has...
are two major brand names for this glass, used both in laboratory glassware
Laboratory glassware
Laboratory glassware refers to a variety of equipment, traditionally made of glass, used for scientific experiments and other work in science, especially in chemistry and biology laboratories...
and in consumer cookware and bakeware
Cookware and bakeware
Cookware and bakeware are types of food preparation containers commonly found in the kitchen. Cookware comprises cooking vessels, such as saucepans and frying pans, intended for use on a stove or range cooktop. Bakeware comprises cooking vessels intended for use inside an oven...
, chiefly for this resistance.
Boron filaments are high-strength, lightweight materials that are used chiefly for advanced aerospace
Aerospace
Aerospace comprises the atmosphere of Earth and surrounding space. Typically the term is used to refer to the industry that researches, designs, manufactures, operates, and maintains vehicles moving through air and space...
structures as a component of composite material
Composite material
Composite materials, often shortened to composites or called composition materials, are engineered or naturally occurring materials made from two or more constituent materials with significantly different physical or chemical properties which remain separate and distinct at the macroscopic or...
s, as well as limited production consumer and sporting goods such as golf club
Golf club (equipment)
A golf club is used to hit a golf ball in a game of golf. Each club is composed of a shaft with a grip and a clubhead. Woods are mainly used for long-distance fairway or tee shots; irons, the most versatile class, are used for a variety of shots; Hybrids that combine design elements of woods and...
s and fishing rod
Fishing rod
A fishing rod or a fishing pole is a tool used to catch fish, usually in conjunction with the pastime of angling, and can also be used in competition casting. . A length of fishing line is attached to a long, flexible rod or pole: one end terminates in a hook for catching the fish...
s. The fibers can be produced by chemical vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
of boron on a tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
filament.
Boron fibers and sub-millimeter sized crystalline boron springs are produced by laser
Laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of photons. The term "laser" originated as an acronym for Light Amplification by Stimulated Emission of Radiation...
-assisted chemical vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
. Translation of the focused laser beam allows to produce even complex helical structures. Such structures show good mechanical properties (elastic modulus
Elastic modulus
An elastic modulus, or modulus of elasticity, is the mathematical description of an object or substance's tendency to be deformed elastically when a force is applied to it...
450 GPa, fracture strain 3.7%, fracture stress 17 GPa) and can be applied as reinforcement of ceramics or in micromechanical systems
Microelectromechanical systems
Microelectromechanical systems is the technology of very small mechanical devices driven by electricity; it merges at the nano-scale into nanoelectromechanical systems and nanotechnology...
.
Detergent formulations and bleaching agents
Borax is used in various household laundry and cleaning products, including the well-known "20 Mule Team Borax" laundry booster and "BoraxoBoraxo
Boraxo is an American brand of powdered hand soap.As its name implies, Boraxo is composed largely of borax, and is marketed as a product for those who get their hands especially dirty during the course of their work, such as mechanics and farmers....
" powdered hand soap. It is also present in some tooth bleaching
Tooth bleaching
Dental bleaching, also known as tooth whitening, is a common procedure in general dentistry but most especially in the field of cosmetic dentistry. A child's deciduous teeth are generally whiter than the adult teeth that follow. As a person ages the adult teeth often become darker due to changes in...
formulas.
Sodium perborate
Sodium perborate
Sodium perborate is a white, odorless, water-soluble chemical compound with the chemical composition 3. It crystallizes as the monohydrate, NaBO3·H2O, trihydrate, NaBO3·3H2O and tetrahydrate, NaBO3·4H2O. The monohydrate and tetrahydrate are the commercially important forms...
serves as a source of active oxygen in many detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s, laundry detergent
Laundry detergent
Laundry detergent, or washing powder, is a substance that is a type of detergent that is added for cleaning laundry. In common usage, "detergent" refers to mixtures of chemical compounds including alkylbenzenesulfonates, which are similar to soap but are less affected by "hard water." In most...
s, cleaning products, and laundry bleaches. However, despite its name, "Borateem" laundry bleach no longer contains any boron compounds, using sodium percarbonate
Sodium percarbonate
Sodium percarbonate is a chemical, an adduct of sodium carbonate and hydrogen peroxide , with formula Na2CO3 · 1.5H2O2. It is a colorless, crystalline, hygroscopic and water-soluble solid...
instead as a bleaching agent.
Insecticides
Boric acid is used as an insecticide, notably against ants, fleas, and cockroaches.Semiconductors
Boron is a useful dopantDopant
A dopant, also called a doping agent, is a trace impurity element that is inserted into a substance in order to alter the electrical properties or the optical properties of the substance. In the case of crystalline substances, the atoms of the dopant very commonly take the place of elements that...
for such semiconductors as silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
, germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
, and silicon carbide
Silicon carbide
Silicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
. Having one fewer valence electron than the host atom, it donates a hole
Electron hole
An electron hole is the conceptual and mathematical opposite of an electron, useful in the study of physics, chemistry, and electrical engineering. The concept describes the lack of an electron at a position where one could exist in an atom or atomic lattice...
resulting in p-type
P-type semiconductor
A P-type semiconductor is obtained by carrying out a process of doping: that is, adding a certain type of atoms to the semiconductor in order to increase the number of free charge carriers ....
conductivity. Traditional method of introducing boron into semiconductors is via its atomic diffusion
Atomic diffusion
Atomic diffusion is a diffusion process whereby the random thermally-activated movement of atoms in a solid results in the net transport of atoms. For example, helium atoms inside a balloon can diffuse through the wall of the balloon and escape, resulting in the balloon slowly deflating. Other air...
at high temperatures. This process uses either solid (B2O3), liquid (BBr3), or gaseous boron sources (B2H6 or BF3). However, after 1970s, it was mostly replaced by ion implantation
Ion implantation
Ion implantation is a materials engineering process by which ions of a material are accelerated in an electrical field and impacted into another solid. This process is used to change the physical, chemical, or electrical properties of the solid...
, which relies mostly on BF3 as a boron source. Boron trichloride gas is also an important chemical in semiconductor industry, however not for doping but rather for plasma etching
Plasma etching
Plasma etching is a form of plasma processing used to fabricate integrated circuits. It involves a high-speed stream of glow discharge of an appropriate gas mixture being shot at a sample. The plasma source, known as etch species, can be either charged or neutral...
of metals and their oxides. Triethylborane is also injected into vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
reactors as a boron source. Examples are the plasma deposition of boron-containing hard carbon films, silicon nitride-boron nitride films, and for doping
Doping (semiconductor)
In semiconductor production, doping intentionally introduces impurities into an extremely pure semiconductor for the purpose of modulating its electrical properties. The impurities are dependent upon the type of semiconductor. Lightly and moderately doped semiconductors are referred to as extrinsic...
of diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
film with boron.
Magnets
Boron is a component of neodymium magnetNeodymium magnet
A neodymium magnet , the most widely-used type of rare-earth magnet, is a permanent magnet made from an alloy of neodymium, iron, and boron to form the Nd2Fe14B tetragonal crystalline structure. Developed in 1982 by General Motors and Sumitomo Special Metals, neodymium magnets are the strongest...
s (Nd2Fe14B), which are the strongest type of permanent magnet. They are found in a variety of domestic and professional electromechanical and electronic devices, such as magnetic resonance imaging
Magnetic resonance imaging
Magnetic resonance imaging , nuclear magnetic resonance imaging , or magnetic resonance tomography is a medical imaging technique used in radiology to visualize detailed internal structures...
(MRI), various motors and actuator
Actuator
An actuator is a type of motor for moving or controlling a mechanism or system. It is operated by a source of energy, usually in the form of an electric current, hydraulic fluid pressure or pneumatic pressure, and converts that energy into some kind of motion. An actuator is the mechanism by which...
s, computer HDDs, CD and DVD players, mobile phones, timer switches, speakers, and so on.
High-hardness and abrasive compounds

Boron carbide and cubic boron nitride powders are widely used as abrasives. Metal boride
Boride
In chemistry a boride is a chemical compound between boron and a less electronegative element, for example silicon boride . The borides are a very large group of compounds that are generally high melting and are not ionic in nature. Some borides exhibit very useful physical properties. The term...
s are used for coating tools through chemical vapor deposition
Chemical vapor deposition
Chemical vapor deposition is a chemical process used to produce high-purity, high-performance solid materials. The process is often used in the semiconductor industry to produce thin films. In a typical CVD process, the wafer is exposed to one or more volatile precursors, which react and/or...
or physical vapor deposition
Physical vapor deposition
Physical vapor deposition is a variety of vacuum deposition and is a general term used to describe any of a variety of methods to deposit thin films by the condensation of a vaporized form of the desired film material onto various workpiece surfaces...
. Implantation of boron ions into metals and alloys, through ion implantation
Ion implantation
Ion implantation is a materials engineering process by which ions of a material are accelerated in an electrical field and impacted into another solid. This process is used to change the physical, chemical, or electrical properties of the solid...
or ion beam deposition
Ion beam deposition
Ion Beam Deposition is a process of applying materials to a target through the application of an ion beam.thumb|Ion beam deposition setup with mass separator...
, results in a spectacular increase in surface resistance and microhardness. Laser alloying has also been successfully used for the same purpose. These borides are an alternative to diamond coated tools, and their (treated) surfaces have similar properties to those of the bulk boride.
Boron carbide
Boron carbideBoron carbide
Boron carbide is an extremely hard boron–carbon ceramic material used in tank armor, bulletproof vests, and numerous industrial applications...
is a ceramic material which is obtained by decomposing B2O3 with carbon in the electric furnace:
- 2 B2O3 + 7 C → B4C + 6 CO
Boron carbide's stucture is only approximately B4C, and it shows a clear depletion of carbon from this suggested stoichiometric ratio. This is due to its very complex structure. The substance can be seen with empirical formula
Empirical formula
In chemistry, the empirical formula of a chemical compound is the simplest positive integer ratio of atoms of each element present in a compound. An empirical formula makes no reference to isomerism, structure, or absolute number of atoms. The empirical formula is used as standard for most ionic...
B12C3 (i.e., with B12 dodecahedra being a motif), but with less carbon as the suggested C3 units are replaced with B-C chains, and there are smaller (B6) octahedra present as well. (See the article for structural analysis).
The repeating polymer plus semi-crystalline structure of boron carbide gives it great structural strength per weight. It is used in tank armor
Vehicle armour
Military vehicles are commonly armoured to withstand the impact of shrapnel, bullets, missiles, or shells, protecting the personnel inside from enemy fire. Such vehicles include tanks, aircraft, and ships....
, bulletproof vest
Bulletproof vest
A ballistic vest, bulletproof vest or bullet-resistant vest is an item of personal armor that helps absorb the impact from firearm-fired projectiles and shrapnel from explosions, and is worn on the torso...
s, and numerous other structural applications.
Boron carbide's ability to absorb neutrons without forming long-lived radionuclide
Radionuclide
A radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma...
s (especially when doped with extra boron-10) makes the material attractive as an absorbent for neutron radiation arising in nuclear power plants. Nuclear applications of boron carbide include shielding, control rods and shut-down pellets. Within control rods, boron carbide is often powdered, to increase its surface area.
| Material | Diamond | cubic-BC2N | cubic-BC5 | cubic-BN | B4C | ReB2 |
|---|---|---|---|---|---|---|
| Vickers hardness (GPa) | 115 | 76 | 71 | 62 | 38 | 22 |
| Fracture toughness Fracture toughness In materials science, fracture toughness is a property which describes the ability of a material containing a crack to resist fracture, and is one of the most important properties of any material for virtually all design applications. The fracture toughness of a material is determined from the... (MPa m1/2) |
5.3 | 4.5 | 9.5 | 6.8 | 3.5 |
Other superhard boron compounds
- HeterodiamondHeterodiamondHeterodiamond is a superhard material containing boron, carbon, and nitrogen . It is formed at high temperatures and high pressures, e.g., by application of an explosive shock wave to a mixture of diamond and cubic boron nitride. The heterodiamond is a polycrystalline material coagulated with...
(also called BCN); - Boron nitrideBoron nitrideBoron nitride is a chemical compound with chemical formula BN, consisting of equal numbers of boron and nitrogen atoms. BN is isoelectronic to a similarly structured carbon lattice and thus exists in various crystalline forms...
. This material is isoelectronic to carbonCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
. Similar to carbon, it has both hexagonal (soft graphite-like h-BN) and cubic (hard, diamond-like c-BN) forms. h-BN is used as a high temperature component and lubricant. c-BN, also known under commercial name borazonBorazonBorazon is a brand name of a cubic form of boron nitride . It is one of the hardest known materials, along with various forms of diamond and boron nitride. Borazon is a crystal created by heating equal quantities of boron and nitrogen at temperatures greater than 1800 °C at 7 GPa...
, is a superior abrasive. Its hardness is only slightly smaller, but chemical stability is superior to that of diamond. - Rhenium diborideRhenium diborideRhenium diboride is a synthetic superhard material. It was first synthesized in 1962 and re-emerged recently due to hopes of achieving high hardness comparable to that of diamond...
can be produced at ambient pressures, but is rather expensive because of rhenium. The hardness of ReB2 exhibits considerable anisotropyAnisotropyAnisotropy is the property of being directionally dependent, as opposed to isotropy, which implies identical properties in all directions. It can be defined as a difference, when measured along different axes, in a material's physical or mechanical properties An example of anisotropy is the light...
because of its hexagonal layered structure. Its value is comparable to that of tungsten carbideTungsten carbideTungsten carbide is an inorganic chemical compound containing equal parts of tungsten and carbon atoms. Colloquially, tungsten carbide is often simply called carbide. In its most basic form, it is a fine gray powder, but it can be pressed and formed into shapes for use in industrial machinery,...
, silicon carbideSilicon carbideSilicon carbide , also known as carborundum, is a compound of silicon and carbon with chemical formula SiC. It occurs in nature as the extremely rare mineral moissanite. Silicon carbide powder has been mass-produced since 1893 for use as an abrasive...
, titanium diboride or zirconium diborideZirconium diborideZirconium diboride is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an Ultra High Temperature Ceramic with a melting point of 3246 °C...
. - AlMgB14 + TiB2 composites possess high hardness and wear resistance and are used in either bulk form or as coatings for components exposed to high temperatures and wear loads.
Shielding in nuclear reactors
Boron shielding is used as a control for nuclear reactorNuclear reactor
A nuclear reactor is a device to initiate and control a sustained nuclear chain reaction. Most commonly they are used for generating electricity and for the propulsion of ships. Usually heat from nuclear fission is passed to a working fluid , which runs through turbines that power either ship's...
s, taking advantage of its high cross-section for neutron capture.
Other nonmedical uses

- Because of its distinctive green flame, amorphous boron is used in pyrotechnic flaresFlare (pyrotechnic)A flare, also sometimes called a fusee, is a type of pyrotechnic that produces a brilliant light or intense heat without an explosion. Flares are used for signalling, illumination, or defensive countermeasures in civilian and military applications...
.*StarchStarchStarch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
and caseinCaseinCasein is the name for a family of related phosphoprotein proteins . These proteins are commonly found in mammalian milk, making up 80% of the proteins in cow milk and between 60% and 65% of the proteins in human milk....
-based adhesives contain sodium tetraborate decahydrate (Na2B4O7•10 H2O) - Some anti-corrosion systems contain borax.
- Sodium borates are used as a fluxFlux (metallurgy)In metallurgy, a flux , is a chemical cleaning agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time...
for soldering silver and gold and with ammonium chlorideAmmonium chlorideAmmonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
for welding ferrous metals. They are also fire retarding additives to plastics and rubber articles. - Boric acidBoric acidBoric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
(also known as orthoboric acid) H3BO3 is used in the production of textile fiberglass and flat panel displayFlat panel displayFlat panel displays encompass a growing number of electronic visual display technologies. They are far lighter and thinner than traditional television sets and video displays that use cathode ray tubes , and are usually less than thick...
s and in many PVAc and PVOH based adhesives. - TriethylboraneTriethylboraneTriethylborane , also called triethylborine and triethylboron, is an organoborane , a near-colorless to yellowish transparent liquid with pungent ether-like odor. Its chemical formula can be written as C6H15B, or 3B, or 3B, or Et3B.Triethylborane is strongly pyrophoric, igniting spontaneously in...
is a substance which ignites the JP-7JP-7JP-7 is a jet fuel developed by the U.S. Air Force for use in supersonic aircraft because of its high flash point and thermal stability. It is the fuel used in the Pratt & Whitney J58 engines, used in the Lockheed SR-71 Blackbird. The air compression of Mach 3+ cruising flight generates very high...
fuel of the Pratt & Whitney J58Pratt & Whitney J58The Pratt & Whitney J58 was a jet engine used on the Lockheed A-12, and subsequently on the YF-12 and SR-71 Blackbird aircraft. The J58 was a variable cycle engine which functioned as both a turbojet and a fan-assisted ramjet. The J58 was a single-spool turbojet engine with an afterburner...
turbojet/ramjetRamjetA ramjet, sometimes referred to as a stovepipe jet, or an athodyd, is a form of airbreathing jet engine using the engine's forward motion to compress incoming air, without a rotary compressor. Ramjets cannot produce thrust at zero airspeed and thus cannot move an aircraft from a standstill...
engines powering the LockheedLockheed CorporationThe Lockheed Corporation was an American aerospace company. Lockheed was founded in 1912 and later merged with Martin Marietta to form Lockheed Martin in 1995.-Origins:...
SR-71 BlackbirdSR-71 BlackbirdThe Lockheed SR-71 "Blackbird" was an advanced, long-range, Mach 3+ strategic reconnaissance aircraft. It was developed as a black project from the Lockheed A-12 reconnaissance aircraft in the 1960s by the Lockheed Skunk Works. Clarence "Kelly" Johnson was responsible for many of the...
. It was also used to ignite the F-1 EnginesF-1 (rocket engine)The F-1 is a rocket engine developed by Rocketdyne and used in the Saturn V. Five F-1 engines were used in the S-IC first stage of each Saturn V, which served as the main launch vehicle in the Apollo program. The F-1 is still the most powerful single-chamber liquid-fueled rocket engine ever...
on the Saturn VSaturn VThe Saturn V was an American human-rated expendable rocket used by NASA's Apollo and Skylab programs from 1967 until 1973. A multistage liquid-fueled launch vehicle, NASA launched 13 Saturn Vs from the Kennedy Space Center, Florida with no loss of crew or payload...
Rocket utilized by NASANASAThe National Aeronautics and Space Administration is the agency of the United States government that is responsible for the nation's civilian space program and for aeronautics and aerospace research...
's Apollo and SkylabSkylabSkylab was a space station launched and operated by NASA, the space agency of the United States. Skylab orbited the Earth from 1973 to 1979, and included a workshop, a solar observatory, and other systems. It was launched unmanned by a modified Saturn V rocket, with a mass of...
programs from 1967 until 1973. Triethylborane is suitable for this because of its pyrophoric properties, especially the fact that it burns with a very high temperature. Triethylborane is an industrial initiatorRadical initiatorIn chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions . These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such...
in radicalRadical (chemistry)Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
reactions, where it is effective even at low temperatures.
Research areas
Magnesium diborideMagnesium diboride
Magnesium diboride is a simple ionic binary compound that has proven to be an inexpensive and useful superconducting material.Its superconductivity was announced in the journal Nature in March 2001. Its critical temperature of is the highest amongst conventional superconductors...
is an important superconducting material
Superconductivity
Superconductivity is a phenomenon of exactly zero electrical resistance occurring in certain materials below a characteristic temperature. It was discovered by Heike Kamerlingh Onnes on April 8, 1911 in Leiden. Like ferromagnetism and atomic spectral lines, superconductivity is a quantum...
with the transition temperature of 39 K. MgB2 wires are produced with the powder-in-tube process and applied in superconducting magnets.
Amorphous boron is used as a melting point depressant in nickel-chromium braze alloys.
Hexagonal boron nitride
Boron nitride
Boron nitride is a chemical compound with chemical formula BN, consisting of equal numbers of boron and nitrogen atoms. BN is isoelectronic to a similarly structured carbon lattice and thus exists in various crystalline forms...
forms atomically thin layers, which have been used to enhance the electron mobility
Electron mobility
In solid-state physics, the electron mobility characterizes how quickly an electron can move through a metal or semiconductor, when pulled by an electric field. In semiconductors, there is an analogous quantity for holes, called hole mobility...
in graphene
Graphene
Graphene is an allotrope of carbon, whose structure is one-atom-thick planar sheets of sp2-bonded carbon atoms that are densely packed in a honeycomb crystal lattice. The term graphene was coined as a combination of graphite and the suffix -ene by Hanns-Peter Boehm, who described single-layer...
devices. It also forms nanotubular structures (BNNTs), which have with high strength, high chemical stability, and high thermal conductivity
Thermal conductivity
In physics, thermal conductivity, k, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction....
, among its list of desirable properties.
Biological role
There is a boron-containing natural antibioticAntibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
, boromycin
Boromycin
Boromycin is a bacteriocidal polyether-macrolide antibiotic. It was initially isolated from the Streptomyces strain Streptomyces antibioticus, and is notable for being the first natural product found to contain the element boron. It is effective against most Gram-positive bacteria, but is...
, isolated from streptomyces
Streptomyces
Streptomyces is the largest genus of Actinobacteria and the type genus of the family Streptomycetaceae. Over 500 species of Streptomyces bacteria have been described. As with the other Actinobacteria, streptomycetes are gram-positive, and have genomes with high guanine and cytosine content...
. Boron is an essential plant nutrient
Nutrient
A nutrient is a chemical that an organism needs to live and grow or a substance used in an organism's metabolism which must be taken in from its environment. They are used to build and repair tissues, regulate body processes and are converted to and used as energy...
, required primarily for maintaining the integrity of cell walls. Conversely, high soil concentrations of > 1.0 ppm
Parts-per notation
In science and engineering, the parts-per notation is a set of pseudo units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement...
can cause marginal and tip necrosis in leaves as well as poor overall growth performance. Levels as low as 0.8 ppm can cause these same symptoms to appear in plants particularly sensitive to boron in the soil. Nearly all plants, even those somewhat tolerant of boron in the soil, will show at least some symptoms of boron toxicity when boron content in the soil is greater than 1.8 ppm. When this content exceeds 2.0 ppm, few plants will perform well and some may not survive. When boron levels in plant tissue exceed 200 ppm symptoms of boron toxicity are likely to appear.
As an ultratrace element
Ultratrace element
In biochemistry, an ultratrace element is a chemical element that normally comprises less than one microgram per gram of a given organism , but which plays a significant role in its metabolism....
, boron is necessary for the optimal health of rats, although it is necessary in such small amounts that ultrapurified foods and dust filtration of air is necessary to induce boron deficiency, which manifest as poor coat or hair quality. Presumably, boron is necessary to other mammals. No deficiency syndrome in humans has been described. Small amounts of boron occur widely in the diet, and the amounts needed in the diet would, by analogy with rodent studies, be very small. The exact physiological role of boron in the animal kingdom is poorly understood.
Boron occurs in all foods produced from plants. Since 1989 its nutritional value has been argued. It is thought that boron plays several biochemical roles in animals, including humans.
The U.S. Department of agriculture conducted an experiment in which postmenopausal women took 3 mg of boron a day. The results showed that supplemental boron reduced excretion of calcium by 44%, and activated estrogen
Estrogen
Estrogens , oestrogens , or œstrogens, are a group of compounds named for their importance in the estrous cycle of humans and other animals. They are the primary female sex hormones. Natural estrogens are steroid hormones, while some synthetic ones are non-steroidal...
and vitamin D
Vitamin D
Vitamin D is a group of fat-soluble secosteroids. In humans, vitamin D is unique both because it functions as a prohormone and because the body can synthesize it when sun exposure is adequate ....
, suggesting a possible role in the suppression of osteoporosis
Osteoporosis
Osteoporosis is a disease of bones that leads to an increased risk of fracture. In osteoporosis the bone mineral density is reduced, bone microarchitecture is deteriorating, and the amount and variety of proteins in bone is altered...
. However, whether these effects were conventionally nutritional, or medicinal, could not be determined. The U.S. National Institutes of Health
National Institutes of Health
The National Institutes of Health are an agency of the United States Department of Health and Human Services and are the primary agency of the United States government responsible for biomedical and health-related research. Its science and engineering counterpart is the National Science Foundation...
states that "Total daily boron intake in normal human diet
Diet (nutrition)
In nutrition, diet is the sum of food consumed by a person or other organism. Dietary habits are the habitual decisions an individual or culture makes when choosing what foods to eat. With the word diet, it is often implied the use of specific intake of nutrition for health or weight-management...
s ranges from 2.1–4.3 mg boron/day."
Analytical quantification
For determination of boron content in food or materials the colorimetricColorimetry
Colorimetry is "the science and technology used to quantify and describe physically the human color perception."It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color perception, most often the CIE 1931 XYZ color space...
curcumin method is used. Boron has to be transferred to boric acid or borate
Borate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
s and on reaction with curcumin
Curcumin
Curcumin is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family . The other two curcuminoids are desmethoxycurcumin and bis-desmethoxycurcumin. The curcuminoids are natural phenols and are responsible for the yellow color of turmeric...
in acidic solution, a red colored boron-chelate complex, rosocyanine
Rosocyanine
Rosocyanine and Rubrocurcumin are two red colored materials, which are formed by the reaction between curcumin and borates.-Application:The color reaction between borates and curcumin is used within the spectrophotometrical determination and quantification of boron present in food or materials...
, is formed.
Boron pharmaceuticals and biologicals
Boric acid has antiseptic, antifungal, and antiviral properties and for this reasons is applied as a water clarifier in swimming pool water treatment. Mild solutions of boric acid have been used as eye antiseptics.A number of potential boronated pharmaceuticals using boron-10, have been prepared for use in boron neutron capture therapy
Boron Neutron Capture Therapy
Boron neutron capture therapy is an experimental form of radiotherapy that uses a neutron beam that interacts with boron injected into a patient...
(BNCT).
Some boron compounds show promise in treating arthritis
Arthritis
Arthritis is a form of joint disorder that involves inflammation of one or more joints....
, though none have as yet been generally approved for the purpose.
Boron is used as an intermediate in pharmaceutical synthesis, but it appeared as an active element in its first-approved organic pharamaceutical in bortezomib
Bortezomib
Bortezomib is the first therapeutic proteasome inhibitor to be tested in humans. It is approved in the U.S. for treating relapsed multiple myeloma and mantle cell lymphoma...
, a new class of drug called proteasome inhibitors, which are active in myeloma and one form of lymphoma. The boron atom in bortezomib binds the catalytic site of the 26S proteasome
Proteasome
Proteasomes are very large protein complexes inside all eukaryotes and archaea, and in some bacteria. In eukaryotes, they are located in the nucleus and the cytoplasm. The main function of the proteasome is to degrade unneeded or damaged proteins by proteolysis, a chemical reaction that breaks...
with high affinity and specificity.
Health issues
Elemental boron, boron oxideBoron trioxide
Boron trioxide is one of the oxides of boron. It is a white, glassy solid with the formula B2O3. It is almost always found as the vitreous form; however, it can be crystallized after extensive annealing...
, boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
, borates, and many organoboron compounds are non-toxic to humans and animals (approximately similar to table salt). The LD50 (dose at which there is 50% mortality) for animals is about 6 g per kg of body weight. Substances with LD50 above 2 g are considered non-toxic. The minimum lethal dose for humans has not been established, but an intake of 4 g/day was reported without incidents, and medical dosages of 20 g of boric acid for neutron capture therapy caused no problems. Fish have survived for 30 min in a saturated boric acid solution and can survive longer in strong borax solutions. Boric acid is more toxic to insects than to mammals, and is routinely used as an insecticide.
The boranes and similar gaseous compounds are quite poisonous. As usual, it is not an element that is intrinsically poisonous, but toxicity depends on structure.
The borane
Borane
In chemistry, a borane is a chemical compound of boron and hydrogen. The boranes comprise a large group of compounds with the generic formulae of BxHy. These compounds do not occur in nature. Many of the boranes readily oxidise on contact with air, some violently. The parent member BH3 is called...
s (boron hydrogen compounds) are toxic as well as highly flammable and require special care when handling. Sodium borohydride presents a fire hazard due to its reducing nature, and the liberation of hydrogen on contact with acid. Boron halides are corrosive.
Congenital endothelial dystrophy type 2
Congenital endothelial dystrophy type 2
Congenital endothelial dystrophy type 2 is a rare form of human corneal dystrophy. It is associated with mutations in SLC4A11 gene.-See also:*Congenital endothelial dystrophy type 1...
, a rare form of corneal dystrophy
Corneal dystrophy (human)
Human corneal dystrophy is a group of disorders, characterised by a noninflammatory, inherited, bilateral opacity of the transparent front part of the human eye called the cornea...
, is linked to mutations in SLC4A11
SLC4A11
Sodium bicarbonate transporter-like protein 11 is a protein that in humans is encoded by the SLC4A11 gene.-Further reading:...
gene that encodes a transporter reportedly regulating the intracellular concentration of boron.
See also
- Allotropes of boronAllotropes of boronElemental boron can exist in several allotropes, the most common of which are crystalline boron and brown amorphous boron. Crystalline boron has four major polymorphs: α, β, γ and T...
- :Category:Boron compounds
- Boron deficiencyBoron deficiency (medicine)Boron deficiency is a pathology which may occur in animals due to a lack of boron. A report given by E. Wayne Johnson et al. at the 2005 Alan D. Leman Swine Conference suggests that boron deficiency produces osteochondrosis in swine that is correctable by addition of 50 ppm of boron to the diet...
- Boron oxide
- Boron nitrideBoron nitrideBoron nitride is a chemical compound with chemical formula BN, consisting of equal numbers of boron and nitrogen atoms. BN is isoelectronic to a similarly structured carbon lattice and thus exists in various crystalline forms...
- Boron neutron capture therapyBoron Neutron Capture TherapyBoron neutron capture therapy is an experimental form of radiotherapy that uses a neutron beam that interacts with boron injected into a patient...
- Boronic acidBoronic acidA boronic acid is an alkyl or aryl substituted boric acid containing a carbon–boron bond belonging to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic...
- Hydroboration-oxidation reactionHydroboration-oxidation reactionIn organic chemistry, the hydroboration–oxidation reaction is a two-step organic reaction that converts an alkene into a neutral alcohol by the net addition of water across the double bond. The hydrogen and hydroxyl group are added in a syn addition leading to cis stereochemistry...
- Suzuki coupling

