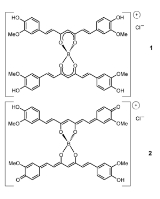
Rosocyanine
Encyclopedia
Rosocyanine and Rubrocurcumin
are two red colored materials, which are formed by the reaction between curcumin
and borate
s.
between borate
s and curcumin
is used within the spectrophotometrical determination and quantification of boron
present in food or materials. Curcumin
is a yellow coloring natural pigment found in the root stocks of some Curcuma
species, especially Curcuma longa
(turmeric), in concentrations up to 3%. In the so called curcumin method for boron quantification it serves as reaction partner for boric acid
. The reaction is very sensitive and so the smallest quantities of boron
can be detected. The maximum absorbance at 540 nm for rosocyanine is used in this colorimetric
method. The formation of rosocyanine depends on the reaction conditions. The reaction is carried out preferentially in acidic solutions containing hydrochloric or sulfuric acid. The color reaction also takes place under different conditions; however, in alkaline solution, gradual decomposition is observed. The reaction might be disturbed at higher pH
values, interfering with other compounds.
 Rosocyanine is formed as 2:1 complex from curcumin and boric acid in acidic solutions. The boron complexes formed with rosocyanine are dioxaborines (here a 1,3,2-dioxaborine). Curcumin possesses a 1,3-diketone
Rosocyanine is formed as 2:1 complex from curcumin and boric acid in acidic solutions. The boron complexes formed with rosocyanine are dioxaborines (here a 1,3,2-dioxaborine). Curcumin possesses a 1,3-diketone
structure and can therefore considered as a chelating agent. Unlike the simpler 1,3-diketone–containing compound acetylacetone
(which forms acetylacetonate complexes
with metals), the entire skeleton of curcumin is in resonance
with the 1,3-dicarbonyl section, making the backbone an extended conjugated system
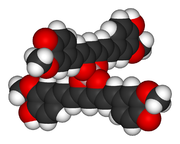
. Investigations of the structure have shown that the positive charge is distributed throughout the molecule. In rosocyanine, the two curcumin moieties are not coplanar but rather perpendicular relative to one another (as seen in the 3D model), as a result of the tetrahedral geometry of tetracoordinate boron. The same applies to rubrocurcumin.
In order to exclude the presence of other materials during the boron quantification using the curcumin method, a variant of the process was developed. In this process, 2,2-dimethyl-1,3-hexanediol or 2-ethyl-1,3-hexanediol are added, in addition to curcumin, to a neutral solution of the boron-containing solution. The complex formed between boron and the 1,3-hexanediol derivative is removed from the aqueous solution by extraction in an organic solvent. Acidification of the organic phase yields rubrocyanine, which can be detected by colorimetric methods. The reaction of curcumin with borates in presence of oxalic acid produces the coloring compound rubrocurcumin
.
, and somewhat soluble (approximately 1%) in pyridine
, sulfuric acid
, and acetic acid
. An alcoholic solution of rosocyanine temporarily turns deeply blue on treatment with alkali
.
In rubrocurcumin one molecule curcumin is replaced with oxalic acid
. Rubrocurcumin produces a similar red colored solution. Rosocyanine is an ionic compound, while rubrocurcumin is a neutral complex.
Rubrocurcumin
Rubrocurcumin is a red colored dye that is formed by the reaction of curcumin and borates.- Synthesis :The reaction of curcumin with borates in presence of oxalic acid produces rubrocurcumin.- Characteristics :...
are two red colored materials, which are formed by the reaction between curcumin
Curcumin
Curcumin is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family . The other two curcuminoids are desmethoxycurcumin and bis-desmethoxycurcumin. The curcuminoids are natural phenols and are responsible for the yellow color of turmeric...
and borate
Borate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
s.
Application
The color reactionColor reaction
In chemistry, a color reaction or colour reaction is a chemical reaction that is used to transform colorless chemical compounds into colored derivatives which can be detected visually or with the aid of a colorimeter....
between borate
Borate
Borates are chemical compounds which contain oxoanions of boron in oxidation state +3. The simplest borate ion, BO33−, has a trigonal planar structure. Other borates are made up of trigonal BO3 or tetrahedral BO4 structural units, sharing oxygen atoms...
s and curcumin
Curcumin
Curcumin is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family . The other two curcuminoids are desmethoxycurcumin and bis-desmethoxycurcumin. The curcuminoids are natural phenols and are responsible for the yellow color of turmeric...
is used within the spectrophotometrical determination and quantification of boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
present in food or materials. Curcumin
Curcumin
Curcumin is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family . The other two curcuminoids are desmethoxycurcumin and bis-desmethoxycurcumin. The curcuminoids are natural phenols and are responsible for the yellow color of turmeric...
is a yellow coloring natural pigment found in the root stocks of some Curcuma
Curcuma
Curcuma is a genus of about 80 accepted species in the plant family Zingiberaceae that contains such species as turmeric and Siam Tulip. The name comes from Arabic kurkum meaning "turmeric". Since assembly of the genus Curcuma by Linnaeus in 1753 about 130 species have been described so far...
species, especially Curcuma longa
Turmeric
Turmeric is a rhizomatous herbaceous perennial plant of the ginger family, Zingiberaceae. It is native to tropical South Asia and needs temperatures between 20 °C and 30 °C and a considerable amount of annual rainfall to thrive...
(turmeric), in concentrations up to 3%. In the so called curcumin method for boron quantification it serves as reaction partner for boric acid
Boric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
. The reaction is very sensitive and so the smallest quantities of boron
Boron
Boron is the chemical element with atomic number 5 and the chemical symbol B. Boron is a metalloid. Because boron is not produced by stellar nucleosynthesis, it is a low-abundance element in both the solar system and the Earth's crust. However, boron is concentrated on Earth by the...
can be detected. The maximum absorbance at 540 nm for rosocyanine is used in this colorimetric
Colorimetry
Colorimetry is "the science and technology used to quantify and describe physically the human color perception."It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color perception, most often the CIE 1931 XYZ color space...
method. The formation of rosocyanine depends on the reaction conditions. The reaction is carried out preferentially in acidic solutions containing hydrochloric or sulfuric acid. The color reaction also takes place under different conditions; however, in alkaline solution, gradual decomposition is observed. The reaction might be disturbed at higher pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
values, interfering with other compounds.

Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
structure and can therefore considered as a chelating agent. Unlike the simpler 1,3-diketone–containing compound acetylacetone
Acetylacetone
Acetylacetone is an organic compound that famously exists in two tautomeric forms that rapidly interconvert. The less stable tautomer is a diketone formally named pentane-2,4-dione. The more common tautomer is the enol form. The pair of tautomers rapidly interconvert and are treated as a single...
(which forms acetylacetonate complexes
Metal acetylacetonates
Metal acetylacetonates are coordination complexes derived from the acetylacetonate anion and metal ions, usually transition metals. The ligand acetylacetonate is often abbreviated acac. Typically both oxygen atoms bind to the metal to form a six-membered chelate ring. The simplest complexes have...
with metals), the entire skeleton of curcumin is in resonance
Resonance (chemistry)
In chemistry, resonance or mesomerism is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula...
with the 1,3-dicarbonyl section, making the backbone an extended conjugated system
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
. Investigations of the structure have shown that the positive charge is distributed throughout the molecule. In rosocyanine, the two curcumin moieties are not coplanar but rather perpendicular relative to one another (as seen in the 3D model), as a result of the tetrahedral geometry of tetracoordinate boron. The same applies to rubrocurcumin.
In order to exclude the presence of other materials during the boron quantification using the curcumin method, a variant of the process was developed. In this process, 2,2-dimethyl-1,3-hexanediol or 2-ethyl-1,3-hexanediol are added, in addition to curcumin, to a neutral solution of the boron-containing solution. The complex formed between boron and the 1,3-hexanediol derivative is removed from the aqueous solution by extraction in an organic solvent. Acidification of the organic phase yields rubrocyanine, which can be detected by colorimetric methods. The reaction of curcumin with borates in presence of oxalic acid produces the coloring compound rubrocurcumin
Rubrocurcumin
Rubrocurcumin is a red colored dye that is formed by the reaction of curcumin and borates.- Synthesis :The reaction of curcumin with borates in presence of oxalic acid produces rubrocurcumin.- Characteristics :...
.
Characteristics
Rosocyanine is a dark green solid with a glossy, metallic shine that forms red colored solutions. It is almost insoluble in water and some organic solvents, very slightly soluble (up to 0.01%) in ethanolEthanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, and somewhat soluble (approximately 1%) in pyridine
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one C-H group replaced by a nitrogen atom...
, sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, and acetic acid
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
. An alcoholic solution of rosocyanine temporarily turns deeply blue on treatment with alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
.
In rubrocurcumin one molecule curcumin is replaced with oxalic acid
Oxalic acid
Oxalic acid is an organic compound with the formula H2C2O4. This colourless solid is a dicarboxylic acid. In terms of acid strength, it is about 3,000 times stronger than acetic acid. Oxalic acid is a reducing agent and its conjugate base, known as oxalate , is a chelating agent for metal cations...
. Rubrocurcumin produces a similar red colored solution. Rosocyanine is an ionic compound, while rubrocurcumin is a neutral complex.

