Allylic rearrangement
Encyclopedia
An allylic rearrangement or allylic shift is an organic reaction
in which the double bond
in an allyl
chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution
.
In reaction conditions that favor a SN1 reaction
mechanism the intermediate is a carbocation
for which several resonance structures are possible. This explains the product distribution (or product spread) after recombination with nucleophile
Y. This type of process is called an SN1' substitution.
Alternatively, it is possible for nucleophile to attack directly at the allylic position, displacing the leaving group in a single step, in a process referred to as SN2' substitution. This is likely in cases when the allyl compound is unhindered, and a strong nucleophile
is used. The products will be similar to those seen with SN1' substitution. Thus reaction of 1-chloro-2-butene with sodium hydroxide gives a mixture of 2-buten-1-ol and 1-buten-3-ol:
Nevertheless, the product in which the OH group is on the primary atom is minor. In the substitution of 1-chloro-3-methyl-2-butene, the tertiary 2-methyl-3-buten-2-ol is produced in a yield of 85%, while that for the primary 3-methyl-2-buten-1-ol is 15%.
In one reaction mechanism
the nucleophile
attacks not directly at the electrophilic site but in a conjugate addition over the double bond:
In the first step of this macrocyclization
the thiol
group in one end of 1,5-pentanedithiol reacts with the butadiene tail in 1 to the enone
2 in an allylic shift with a sulfone
leaving group
which reacts further with the other end in a conjugate addition reaction.
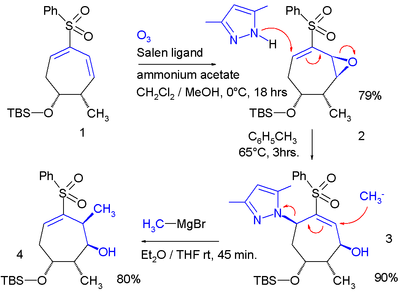
In one study the allylic shift was applied twice in a ring system:
In this reaction sequence a Jacobson epoxidation adds an epoxy
group to a diene
which serves as the leaving group in reaction with the pyrazole
nucleophile. The second nucleophile is methylmagnesium bromide expulsing the pyrazole group.
An SN2' reaction should explain the outcome of the reaction of an aziridine
carrying a methylene bromide group with methyllithium :
In this reaction one equivalent of acetylene
is lost.
Examples of allylic shifts:
is accompanied by a rearrangement. One example of such reaction is found as part of a Taxol total synthesis
(ring C):
The hydride
is lithium aluminium hydride
and the leaving group a phosphonium salt
. The product contains a new exocyclic double bond. Only when the cyclohexane
ring is properly substituted will the proton add in a trans position with respect to the adjacent methyl group. A conceptually related reaction is the Whiting reaction
forming dienes.
s. In the example below the carbonyl
group in benzaldehyde
is activated by diboronic acid prior to reaction with the allyl alcohol (see: Prins reaction
):
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in which the double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
in an allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution
Nucleophilic substitution
In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive...
.
In reaction conditions that favor a SN1 reaction
SN1 reaction
The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular...
mechanism the intermediate is a carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
for which several resonance structures are possible. This explains the product distribution (or product spread) after recombination with nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
Y. This type of process is called an SN1' substitution.
Alternatively, it is possible for nucleophile to attack directly at the allylic position, displacing the leaving group in a single step, in a process referred to as SN2' substitution. This is likely in cases when the allyl compound is unhindered, and a strong nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
is used. The products will be similar to those seen with SN1' substitution. Thus reaction of 1-chloro-2-butene with sodium hydroxide gives a mixture of 2-buten-1-ol and 1-buten-3-ol:
Nevertheless, the product in which the OH group is on the primary atom is minor. In the substitution of 1-chloro-3-methyl-2-butene, the tertiary 2-methyl-3-buten-2-ol is produced in a yield of 85%, while that for the primary 3-methyl-2-buten-1-ol is 15%.
In one reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
the nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
attacks not directly at the electrophilic site but in a conjugate addition over the double bond:
Scope
The synthetic utility can be extended to substitutions over butadiene bonds: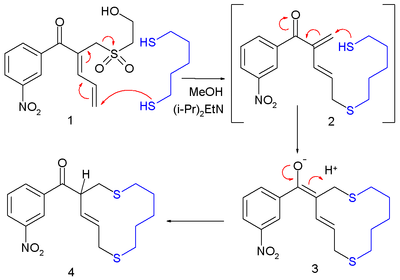
- Reaction in methanolMethanolMethanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
and catalyst diisopropylethylamine
In the first step of this macrocyclization
Macrocycle
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule." In the chemical literature, organic chemists may consider any molecule containing a ring of nine or more atoms to be a macrocycle...
the thiol
Thiol
In organic chemistry, a thiol is an organosulfur compound that contains a carbon-bonded sulfhydryl group...
group in one end of 1,5-pentanedithiol reacts with the butadiene tail in 1 to the enone
Enone
An enone is an unsaturated chemical compound or functional group consisting of a conjugated system of an alkene and a ketone. The simplest enone is methyl vinyl ketone or CH2=CHCOCH3....
2 in an allylic shift with a sulfone
Sulfone
A sulfone is a chemical compound containing a sulfonyl functional group attached to two carbon atoms. The central hexavalent sulfur atom is double bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents.-IUPAC name and...
leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
which reacts further with the other end in a conjugate addition reaction.
In one study the allylic shift was applied twice in a ring system:
In this reaction sequence a Jacobson epoxidation adds an epoxy
Epoxy
Epoxy, also known as polyepoxide, is a thermosetting polymer formed from reaction of an epoxide "resin" with polyamine "hardener". Epoxy has a wide range of applications, including fiber-reinforced plastic materials and general purpose adhesives....
group to a diene
Diene
In organic chemistry a diene or diolefin is a hydrocarbon that contains two carbon double bonds.Conjugated dienes are functional groups, with a general formula of CnH2n-2. Dienes and alkynes are functional isomers...
which serves as the leaving group in reaction with the pyrazole
Pyrazole
Pyrazole refers both to the class of simple aromatic ring organic compounds of the heterocyclic diazole series characterized by a 5-membered ring structure composed of three carbon atoms and two nitrogen atoms in adjacent positions, and to the unsubstituted parent compound...
nucleophile. The second nucleophile is methylmagnesium bromide expulsing the pyrazole group.
An SN2' reaction should explain the outcome of the reaction of an aziridine
Aziridine
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group and two methylene groups...
carrying a methylene bromide group with methyllithium :
In this reaction one equivalent of acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
is lost.
Examples of allylic shifts:
- Ferrier rearrangementFerrier rearrangementThe Ferrier rearrangement is an organic reaction that involves a nucleophilic substitution reaction combined with an allylic shift in a glycal . It was discovered by the carbohydrate chemist Robert J...
- Meyer–Schuster rearrangement
SN2' reduction
In one adaptation called a SN2' reduction a formal organic reduction on an allyl group containing a good leaving groupLeaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
is accompanied by a rearrangement. One example of such reaction is found as part of a Taxol total synthesis
Taxol total synthesis
Paclitaxel total synthesis in organic chemistry is a major ongoing research effort in the total synthesis of paclitaxel . This diterpenoid is an important drug in the treatment of cancer but also expensive because the compound is harvested from a scarce resource, namely the Pacific yew...
(ring C):
The hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
is lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
and the leaving group a phosphonium salt
Phosphonium salt
A phosphonium salt is a salt containing the phosphonium ion such as phosphonium iodide . More commonly, phosphonium refers to a quaternary organic derivative such as tetraphenylphosphonium chloride, 4P+ Cl- and tetramethylphosphonium iodide, [P4]+I−.Alkyltriphenylphosphonium salts are widely...
. The product contains a new exocyclic double bond. Only when the cyclohexane
Cyclohexane
Cyclohexane is a cycloalkane with the molecular formula C6H12. Cyclohexane is used as a nonpolar solvent for the chemical industry, and also as a raw material for the industrial production of adipic acid and caprolactam, both of which being intermediates used in the production of nylon...
ring is properly substituted will the proton add in a trans position with respect to the adjacent methyl group. A conceptually related reaction is the Whiting reaction
Whiting reaction
The Whiting reaction is an organic reaction converting a propargyl diol into a diene using lithium aluminium hydride.This organic reduction has been applied in the synthesis of fecapentaene....
forming dienes.
Electrophilic allyl shifts
Allyl shifts can also take place with electrophileElectrophile
In general electrophiles are positively charged species that are attracted to an electron rich centre. In chemistry, an electrophile is a reagent attracted to electrons that participates in a chemical reaction by accepting an electron pair in order to bond to a nucleophile...
s. In the example below the carbonyl
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups....
group in benzaldehyde
Benzaldehyde
Benzaldehyde is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful. This colorless liquid has a characteristic pleasant almond-like odor...
is activated by diboronic acid prior to reaction with the allyl alcohol (see: Prins reaction
Prins reaction
The Prins reaction is an organic reaction consisting of an electrophilic addition of an aldehyde or ketone to an alkene or alkyne followed by capture of a nucleophile. The outcome of the reaction depends on reaction conditions . With water and a protic acid such as sulfuric acid as the reaction...
):