
Thiol
Encyclopedia
In organic chemistry, a thiol (icon) is an organosulfur compound that contains a carbon-bonded sulfhydryl (–C–SH or R–SH) group (where R represents an alkane, alkene, or other carbon-containing group of atoms). Thiols are the sulfur analogue of alcohol
s (that is, sulfur takes the place of oxygen in the hydroxyl group of an alcohol), and the word is a portmanteau of "thio" + "alcohol," with the first word deriving from Greek θεῖον ("thion") = "sulfur".
The –SH functional group itself is referred to as either a thiol group or a sulfhydryl group.
Many thiols have strong odors resembling that of garlic, and indeed the odor of garlic itself is due to a thiol. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant.
Thiols are often referred to as mercaptans. The term mercaptan is derived from the Latin
mercurium captans (capturing mercury) because the thiolate group bonds so strongly with mercury
compounds.
, C–S bond lengths being around 180 picometers in length. The C–S–H angles approach 90°. In the solid or molten liquids, the hydrogen-bonding between individual thiol groups is weak, the main cohesive force being van der Waals interactions between the highly polarizable divalent sulfur centers.
Due to the lesser electronegativity
difference between sulfur and hydrogen compared to oxygen and hydrogen, an S–H bond is less polar
than the hydroxyl group. Thiols have a lower dipole moment
relative to the corresponding alcohol.
s resembling that of garlic
. The odors of thiols are often strong and repulsive, particularly for those of low molecular weight. The spray of skunk
s consists mainly of low-molecular-weight thiol compounds. These compounds are detectable by the human nose at concentrations of only 10 parts per billion.
Thiols are also responsible for a class of wine fault
s caused by an unintended reaction between sulfur and yeast
and the "skunky" odor of beer that has been exposed to ultraviolet light.
Not all thiols have unpleasant odors. For example, grapefruit mercaptan, a monoterpenoid
thiol, is responsible for the characteristic scent of grapefruit
. This effect is present only at low concentrations. The pure mercaptan has an unpleasant odor.
Natural gas
distributors began adding thiols, originally ethanethiol
, to natural gas
, which is naturally odorless, after the deadly New London School explosion
in New London, Texas
, in 1937. Most gas odorants utilized currently contain mixtures of mercaptans and sulfides, with t-butyl mercaptan
as the main odor constituent. In situations where thiols are used in commercial industry, such as liquid petroleum gas tankers and bulk handling systems, an oxidizing catalyst is used to destroy the odor. A copper-based oxidation catalyst neutralizes the volatile thiols and transforms them into inert products.
ing, with both water molecules and among themselves. Hence, they have lower boiling point
s and are less soluble in water and other polar solvents than alcohols of similar molecular weight. Thiols and thioethers have similar solubility characteristics and boiling points.
s"). The νSH band appears near 2400 cm−1 in the IR spectrum. In a colorimetric test, thiols react with nitroprusside.
with the methanol. This method is employed for the industrial synthesis of methanethiol
:
Such reactions are conducted in the presence of acidic catalysts. The other principal route to thiols involves the addition of hydrogen sulfide to alkenes. Such reactions are usually conducted in the presence of an acid catalyst or UV light. Halide displacement, using the suitable organic halide and sodium hydrogen sulfide has also been utilized.
is generally inefficient owing to the competing formation of thioether
s:
Instead, alkyl halides are converted to thiols via a S-alkylation of thiourea
. This multistep, one-pot process proceeds via the intermediacy of the isothiouronium salt, which is hydrolyzed in a separate step:
The thiourea route works well with primary halides, especially activated ones. Secondary and tertiary thiols are less easily prepared. Secondary thiols can be prepared from the ketone via the corresponding dithioketals.
Organolithium compounds and Grignard reagents react with sulfur to give the thiolates, which are readily hydrolyzed:
Phenols can be converted to the thiophenols via rearrangement of their O-aryl dialkylthiocarbamates.
Many thiols are prepared by reductive dealkylation of thioethers, especially benzyl derivatives and thioacetals.
s, thioacetal
s and thioester
s, which are analogous to ethers, acetal
s, and esters. Thiols and alcohols are also very different in their reactivity, thiols being easily oxidized and thiolates being highly potent nucleophiles.
of 10.5 vs 15 for butanol. Thiophenol has a pKa
of 6 vs 10 for phenol. Thus, thiolates can be obtained from thiols by treatment with alkali hydroxides.
by reagents such as iodine
to give an organic disulfide
(R–S–S–R).
Oxidation by more powerful reagents such as sodium hypochlorite or hydrogen peroxide
yields sulfonic acid
s (RSO3H).
Oxidation by oxygen in the presence of heterogeneous catalysts:
Thiols participate in thiol-disulfide exchange:
This reaction is especially important in nature.
. The term mercaptan is derived from the Latin
mercurium captans (capturing mercury) because the thiolate group bonds so strongly with mercury
compounds. The stability of metal thiolates parallels that of the corresponding sulfide minerals.
and biochemistry
. They have the formula RS where R is an organic substituent such as alkyl or aryl
. They arise from or can be generated by a number of routes, but the principal method is H-atom abstraction from thiols. Another method involves homolysis
of organic disulfides. In biology thiyl radicals are responsible for the formation of the deoxyribonucleic acids, building blocks for DNA
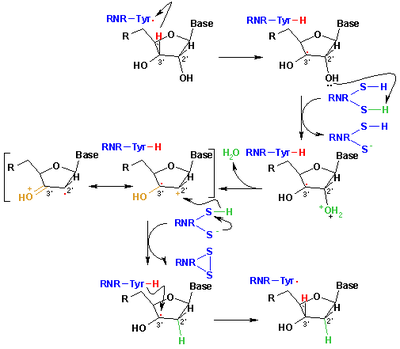
. This conversion is catalysed by ribonucleotide reductase
(see figure). Thiyl intermediates also are produced by the oxidation of glutathione
, an antioxidant in biology. Thiyl radicals are also intermediates in the vulcanization
process. For example, the vulcanization of polyisoprene results when mercapto radicals couple forming disulfide and polysulfide
crosslinks.
cysteine
, the thiol group plays an important role in biology. When the thiol groups of two cysteine residues (as in monomers or constituent units) are brought near each other in the course of protein
folding, an oxidation reaction can generate a cystine
unit with a disulfide bond
(-S-S-). Disulfide bonds can contribute to a protein's tertiary structure
if the cysteines are part of the same peptide
chain, or contribute to the quaternary structure
of multi-unit proteins by forming fairly strong covalent bonds between different peptide chains. A physical manifestation of cysteine-cystine equilibrium is provided by hair straightening
technologies.
Sulfhydryl groups in the active site
of an enzyme
can form noncovalent bonds with the enzyme's substrate
as well, contributing to catalytic activity
. Active site cysteine residues are the functional unit in cysteine protease
s. Cysteine residues may also react with heavy metal ions (Zn2+, Cd2+, Pb2+, Hg2+, Ag+) because of the high affinity between the soft sulfide and the soft metal (see hard and soft acids and bases). This can deform and inactivate the protein, and is one mechanism of heavy metal poisoning
.
. The biosynthesis
of methane
, the principal hydrocarbon
on earth, arises from the reaction mediated by coenzyme M
, 2-mercaptoethyl sulfonic acid.Thiolates, the conjugate bases derived from thiols, form strong complexes with many metal ions, especially those classified as soft. The term mercaptan is derived from the Latin mercurium captans (capturing mercury)[4] because the thiolate group bonds so strongly with mercury compounds. The stability of metal thiolates parallels that of the corresponding sulfide minerals.
Sulfhydryl groups in the active site of an enzyme can form noncovalent bonds with the enzyme's substrate as well, contributing to catalytic activity. Active site cysteine residues are the functional unit in cysteine proteases. Cysteine residues may also react with heavy metal ions (Zn2+, Cd2+, Pb2+, Hg2+, Ag+) because of the high affinity between the soft sulfide and the soft metal (see hard and soft acids and bases). This can deform and inactivate the protein, and is one mechanism of heavy metal poisoning.
[edit]
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s (that is, sulfur takes the place of oxygen in the hydroxyl group of an alcohol), and the word is a portmanteau of "thio" + "alcohol," with the first word deriving from Greek θεῖον ("thion") = "sulfur".
The –SH functional group itself is referred to as either a thiol group or a sulfhydryl group.
Many thiols have strong odors resembling that of garlic, and indeed the odor of garlic itself is due to a thiol. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant.
Thiols are often referred to as mercaptans. The term mercaptan is derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
mercurium captans (capturing mercury) because the thiolate group bonds so strongly with mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
compounds.
Structure and bonding
Thiols and alcohols have similar molecular structure. The major difference is the size of the chalcogenideChalcogenide
A chalcogenide is a chemical compound consisting of at least one chalcogen ion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term is more commonly reserved for sulfides, selenides, and tellurides, rather than...
, C–S bond lengths being around 180 picometers in length. The C–S–H angles approach 90°. In the solid or molten liquids, the hydrogen-bonding between individual thiol groups is weak, the main cohesive force being van der Waals interactions between the highly polarizable divalent sulfur centers.
Due to the lesser electronegativity
Electronegativity
Electronegativity, symbol χ , is a chemical property that describes the tendency of an atom or a functional group to attract electrons towards itself. An atom's electronegativity is affected by both its atomic number and the distance that its valence electrons reside from the charged nucleus...
difference between sulfur and hydrogen compared to oxygen and hydrogen, an S–H bond is less polar
Chemical polarity
In chemistry, polarity refers to a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in...
than the hydroxyl group. Thiols have a lower dipole moment
Bond dipole moment
The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. The bond dipole μ is given by:\mu = \delta \, d....
relative to the corresponding alcohol.
Nomenclature
There are several ways to name the alkylthiols:- The preferred method (used by the IUPAC) is to add the suffix -thiol to the name of the alkane. The method is nearly identical to naming an alcohol. Example: CH3SH would be methanethiolMethanethiolMethanethiol is a colorless gas with a smell like rotten cabbage. It is a natural substance found in the blood and brain of humans and other animal as well as plant tissues. It is disposed of through animal feces. It occurs naturally in certain foods, such as some nuts and cheese...
. - An older method, the word mercaptan replaces alcohol in the name of the equivalent alcohol compound. Example: CH3SH would be methyl mercaptan, just as CH3OH is called methyl alcohol.
- As a prefix, the terms sulfanyl or mercapto are used. Example: mercaptopurineMercaptopurineMercaptopurine is an immunosuppressive drug.It is a thiopurine.-Uses:...
.
Odor
Many thiols have strong odorOdor
An odor or odour is caused by one or more volatilized chemical compounds, generally at a very low concentration, that humans or other animals perceive by the sense of olfaction. Odors are also commonly called scents, which can refer to both pleasant and unpleasant odors...
s resembling that of garlic
Garlic
Allium sativum, commonly known as garlic, is a species in the onion genus, Allium. Its close relatives include the onion, shallot, leek, chive, and rakkyo. Dating back over 6,000 years, garlic is native to central Asia, and has long been a staple in the Mediterranean region, as well as a frequent...
. The odors of thiols are often strong and repulsive, particularly for those of low molecular weight. The spray of skunk
Skunk
Skunks are mammals best known for their ability to secrete a liquid with a strong, foul odor. General appearance varies from species to species, from black-and-white to brown or cream colored. Skunks belong to the family Mephitidae and to the order Carnivora...
s consists mainly of low-molecular-weight thiol compounds. These compounds are detectable by the human nose at concentrations of only 10 parts per billion.
Thiols are also responsible for a class of wine fault
Wine fault
A wine fault or defect is an unpleasant characteristic of a wine often resulting from poor winemaking practices or storage conditions, and leading to wine spoilage. Many of the compounds that cause wine faults are already naturally present in wine but at insufficient concentrations to adversely...
s caused by an unintended reaction between sulfur and yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
and the "skunky" odor of beer that has been exposed to ultraviolet light.
Not all thiols have unpleasant odors. For example, grapefruit mercaptan, a monoterpenoid
Terpene
Terpenes are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, though also by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeterium. They are often strong smelling and thus may have had a protective...
thiol, is responsible for the characteristic scent of grapefruit
Grapefruit
The grapefruit , is a subtropical citrus tree known for its sour fruit, an 18th-century hybrid first bred in Barbados. When found, it was named the "forbidden fruit"; it has also been misidentified with the pomelo or shaddock , one of the parents of this hybrid, the other being sweet orange The...
. This effect is present only at low concentrations. The pure mercaptan has an unpleasant odor.
Natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
distributors began adding thiols, originally ethanethiol
Ethanethiol
Ethanethiol is an organic compound with the formula CH3CH2SH. It consists of an ethyl group, CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with S instead of O. The presence of S leads to many different properties, most notably the infamous odour of EtSH...
, to natural gas
Natural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
, which is naturally odorless, after the deadly New London School explosion
New London School explosion
The New London School explosion occurred on March 18, 1937, when a natural gas leak caused an explosion, destroying the London School of New London, Texas, a community in Rusk County previously known as "London". The disaster killed more than 295 students and teachers, making it the worst...
in New London, Texas
New London, Texas
New London is a city in Rusk County, Texas, United States. The population was 987 at the 2000 census.On March 18, 1937, the London School Explosion killed in excess of three hundred people...
, in 1937. Most gas odorants utilized currently contain mixtures of mercaptans and sulfides, with t-butyl mercaptan
Butanethiol
Butanethiol, also known as butyl mercaptan, is a volatile, clear to yellowish liquid with a fetid odor, commonly described as "skunk" odor. In fact, butanethiol is structurally similar to several major constituents of a skunk's defensive spray...
as the main odor constituent. In situations where thiols are used in commercial industry, such as liquid petroleum gas tankers and bulk handling systems, an oxidizing catalyst is used to destroy the odor. A copper-based oxidation catalyst neutralizes the volatile thiols and transforms them into inert products.
Boiling points and solubility
Thiols show little association by hydrogen bondHydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing, with both water molecules and among themselves. Hence, they have lower boiling point
Boiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
s and are less soluble in water and other polar solvents than alcohols of similar molecular weight. Thiols and thioethers have similar solubility characteristics and boiling points.
Characterization
Volatile thiols are easily and almost unerringly detected by their distinctive odor. S-specific analyzers for gas chromatographs are useful. Spectroscopic indicators are the D2O-exchangeable SH signal in the 1H NMR spectrum (S has no useful "NMR isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s"). The νSH band appears near 2400 cm−1 in the IR spectrum. In a colorimetric test, thiols react with nitroprusside.
Preparation
In industry, methanethiol is prepared by the reaction of hydrogen sulfideHydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
with the methanol. This method is employed for the industrial synthesis of methanethiol
Methanethiol
Methanethiol is a colorless gas with a smell like rotten cabbage. It is a natural substance found in the blood and brain of humans and other animal as well as plant tissues. It is disposed of through animal feces. It occurs naturally in certain foods, such as some nuts and cheese...
:
- CH3OH + H2S → CH3SH + H2O
Such reactions are conducted in the presence of acidic catalysts. The other principal route to thiols involves the addition of hydrogen sulfide to alkenes. Such reactions are usually conducted in the presence of an acid catalyst or UV light. Halide displacement, using the suitable organic halide and sodium hydrogen sulfide has also been utilized.
Laboratory methods
Many methods are useful for the synthesis of thiols on the laboratory scale. The direct reaction of a halogenoalkane with sodium hydrosulfideSodium hydrosulfide
Sodium hydrosulfide is the chemical compound with the formula NaHS. This compound is the product of the half neutralization of hydrogen sulfide with sodium hydroxide. NaHS is a useful reagent for the synthesis of organic and inorganic sulfur compounds. It is a colorless solid that typically smells...
is generally inefficient owing to the competing formation of thioether
Thioether
A thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
s:
- CH3CH2Br + NaSH → CH3CH2SH + NaBr
- CH3CH2Br + CH3CH2SH → (CH3CH2)2S + HBr
Instead, alkyl halides are converted to thiols via a S-alkylation of thiourea
Thiourea
Thiourea is an organosulfur compound of with the formula SC2 . It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refers to a broad...
. This multistep, one-pot process proceeds via the intermediacy of the isothiouronium salt, which is hydrolyzed in a separate step:
- CH3CH2Br + SC(NH2)2 → [CH3CH2SC(NH2)2]Br
- [CH3CH2SC(NH2)2]Br + NaOH → CH3CH2SH + OC(NH2)2 + NaBr
The thiourea route works well with primary halides, especially activated ones. Secondary and tertiary thiols are less easily prepared. Secondary thiols can be prepared from the ketone via the corresponding dithioketals.
Organolithium compounds and Grignard reagents react with sulfur to give the thiolates, which are readily hydrolyzed:
- RLi + S → RSLi
- RSLi + HCl → RSH + LiCl
Phenols can be converted to the thiophenols via rearrangement of their O-aryl dialkylthiocarbamates.
Many thiols are prepared by reductive dealkylation of thioethers, especially benzyl derivatives and thioacetals.
Reactions
Akin to the chemistry of alcohols, thiols form thioetherThioether
A thioether is a functional group in organosulfur chemistry with the connectivity C-S-C as shown on right. Like many other sulfur-containing compounds, volatile thioethers have foul odors. A thioether is similar to an ether except that it contains a sulfur atom in place of the oxygen...
s, thioacetal
Thioacetal
Thioacetals are the sulfur analogue of acetals. They are prepared in a similar way to acetals: by reacting a thiol with an aldehyde:Dithioacetals are prepared similarly to thioacetals, which are intermediates:...
s and thioester
Thioester
Thioesters are compounds with the functional group C-S-CO-C. They are the product of esterification between a carboxylic acid and a thiol. Thioesters are widespread in biochemistry, the best-known derivative being acetyl-CoA.-Synthesis:...
s, which are analogous to ethers, acetal
Acetal
An acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
s, and esters. Thiols and alcohols are also very different in their reactivity, thiols being easily oxidized and thiolates being highly potent nucleophiles.
S-alkylation
Thiols, or more particularly their conjugate bases, are readily alkylated to give thioethers:- RSH + R'Br + base → RSR' + [Hbase]Br
Acidity
Relative to the alcohols, thiols are fairly acidic. Butanethiol has a pKaPKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of 10.5 vs 15 for butanol. Thiophenol has a pKa
PKA
PKA, pKa, or other similar variations may stand for:* pKa, the symbol for the acid dissociation constant at logarithmic scale* Protein kinase A, a class of cAMP-dependent enzymes* Pi Kappa Alpha, the North-American social fraternity...
of 6 vs 10 for phenol. Thus, thiolates can be obtained from thiols by treatment with alkali hydroxides.
Redox
Thiols, especially in the presence of base, are readily oxidizedRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
by reagents such as iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
to give an organic disulfide
Disulfide
In chemistry, a disulfide usually refers to the structural unit composed of a linked pair of sulfur atoms. Disulfide usually refer to a chemical compound that contains a disulfide bond, such as diphenyl disulfide, C6H5S-SC6H5....
(R–S–S–R).
- 2 R–SH + Br2 → R–S–S–R + 2 HBr
Oxidation by more powerful reagents such as sodium hypochlorite or hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
yields sulfonic acid
Sulfonic acid
Sulfonic acid usually refers to a member of the class of organosulfur compounds with the general formula RS2–OH, where R is an alkyl or aryl. The formal part of acid, HS2–OH, are formally derivatives of the "parent" inorganic compound with the formula HSO2.-Preparation:Sulfonic acid is...
s (RSO3H).
- R–SH + 3H2O2 → RSO3H + 3H2O
Oxidation by oxygen in the presence of heterogeneous catalysts:
- 2R–SH + 1/2O2 → RS–SR + H2O
Thiols participate in thiol-disulfide exchange:
- RS–SR + 2 R'SH → 2 RSH + R'S–SR'
This reaction is especially important in nature.
Metal ion complexation
Thiolates, the conjugate bases derived from thiols, form strong complexes with many metal ions, especially those classified as softHSAB theory
The HSAB concept is an acronym for 'hard and soft acids and bases. Also known as the Pearson acid base concept, HSAB is widely used in chemistry for explaining stability of compounds, reaction mechanisms and pathways....
. The term mercaptan is derived from the Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
mercurium captans (capturing mercury) because the thiolate group bonds so strongly with mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
compounds. The stability of metal thiolates parallels that of the corresponding sulfide minerals.
Thiyl radicals
Free radicals derived from mercaptans, called thiyl or thiol radical or mercapto radical, are commonly invoked to explain reactions in organic chemistryOrganic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
and biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
. They have the formula RS where R is an organic substituent such as alkyl or aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
. They arise from or can be generated by a number of routes, but the principal method is H-atom abstraction from thiols. Another method involves homolysis
Homolysis
In general it means breakdown to equal pieces There are separate meanings for the word in chemistry and biology.-Homolysis in chemistry:...
of organic disulfides. In biology thiyl radicals are responsible for the formation of the deoxyribonucleic acids, building blocks for DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
. This conversion is catalysed by ribonucleotide reductase
Ribonucleotide reductase
Ribonucleotide reductase is an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides. Deoxyribonucleotides in turn are used in the synthesis of DNA. The reaction catalyzed by RNR is strictly conserved in all living organisms...
(see figure). Thiyl intermediates also are produced by the oxidation of glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
, an antioxidant in biology. Thiyl radicals are also intermediates in the vulcanization
Vulcanization
Vulcanization or vulcanisation is a chemical process for converting rubber or related polymers into more durable materials via the addition of sulfur or other equivalent "curatives." These additives modify the polymer by forming crosslinks between individual polymer chains. Vulcanized material is...
process. For example, the vulcanization of polyisoprene results when mercapto radicals couple forming disulfide and polysulfide
Polysulfide
Polysulfides are a class of chemical compounds containing chains of sulfur atoms. There are two main classes of polysulfides: anions and organic polysulfides. Anions have the general formula Sn2−. These anions are the conjugate bases of the hydrogen polysulfides H2nSn...
crosslinks.

Cysteine and cystine
As the functional group of the amino acidAmino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
, the thiol group plays an important role in biology. When the thiol groups of two cysteine residues (as in monomers or constituent units) are brought near each other in the course of protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
folding, an oxidation reaction can generate a cystine
Cystine
Cystine is a dimeric amino acid formed by the oxidation of two cysteine residues that covalently link to make a disulfide bond. This organosulfur compound has the formula 2. It is a white solid, and melts at 247-249 °C...
unit with a disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
(-S-S-). Disulfide bonds can contribute to a protein's tertiary structure
Tertiary structure
In biochemistry and molecular biology, the tertiary structure of a protein or any other macromolecule is its three-dimensional structure, as defined by the atomic coordinates.-Relationship to primary structure:...
if the cysteines are part of the same peptide
Peptide
Peptides are short polymers of amino acid monomers linked by peptide bonds. They are distinguished from proteins on the basis of size, typically containing less than 50 monomer units. The shortest peptides are dipeptides, consisting of two amino acids joined by a single peptide bond...
chain, or contribute to the quaternary structure
Quaternary structure
In biochemistry, quaternary structure is the arrangement of multiple folded protein or coiling protein molecules in a multi-subunit complex.-Description and examples:...
of multi-unit proteins by forming fairly strong covalent bonds between different peptide chains. A physical manifestation of cysteine-cystine equilibrium is provided by hair straightening
Hair straightening
Hair straightening is a hair styling technique which involves the flattening and straightening of hair in order to give it a smooth, streamlined, and 'sleek' appearance. It may be accomplished by using hair irons and hot combs, chemical relaxers, Japanese hair straightening, or Brazilian hair...
technologies.
Sulfhydryl groups in the active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
of an enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
can form noncovalent bonds with the enzyme's substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
as well, contributing to catalytic activity
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
. Active site cysteine residues are the functional unit in cysteine protease
Cysteine protease
Proteases are enzymes that degrade polypeptides. Cysteine proteases have a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic dyad. The first step is deprotonation of a thiol in the enzyme's active site by an adjacent amino acid with a basic side chain, usually a...
s. Cysteine residues may also react with heavy metal ions (Zn2+, Cd2+, Pb2+, Hg2+, Ag+) because of the high affinity between the soft sulfide and the soft metal (see hard and soft acids and bases). This can deform and inactivate the protein, and is one mechanism of heavy metal poisoning
Heavy Metal Poisoning
Heavy Metal Poisoning is a song by American rock band Styx. It was included as the fifth track on their 1983 studio album Kilroy Was Here.The song in the story of Kilroy Was Here has the character of Dr Righteous preaching the evils of rock and roll...
.
Cofactors
Many cofactors (non-protein-based helper molecules) feature thiols. The biosynthesis and degradation of fatty acids and related long-chain hydrocarbons is conducted on a scaffold that anchors the growing chain through a thioester derived from the thiol Coenzyme ACoenzyme A
Coenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
. The biosynthesis
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
of methane
Methane
Methane is a chemical compound with the chemical formula . It is the simplest alkane, the principal component of natural gas, and probably the most abundant organic compound on earth. The relative abundance of methane makes it an attractive fuel...
, the principal hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
on earth, arises from the reaction mediated by coenzyme M
Coenzyme M
Coenzyme M is a coenzyme required for methyl-transfer reactions in the metabolism of methanogens. The coenzyme is an anion with the formula . It is named 2-mercaptoethanesulfonate and abbreviated HS–CoM. The cation is unimportant, but the sodium salt is most available...
, 2-mercaptoethyl sulfonic acid.Thiolates, the conjugate bases derived from thiols, form strong complexes with many metal ions, especially those classified as soft. The term mercaptan is derived from the Latin mercurium captans (capturing mercury)[4] because the thiolate group bonds so strongly with mercury compounds. The stability of metal thiolates parallels that of the corresponding sulfide minerals.
Sulfhydryl groups in the active site of an enzyme can form noncovalent bonds with the enzyme's substrate as well, contributing to catalytic activity. Active site cysteine residues are the functional unit in cysteine proteases. Cysteine residues may also react with heavy metal ions (Zn2+, Cd2+, Pb2+, Hg2+, Ag+) because of the high affinity between the soft sulfide and the soft metal (see hard and soft acids and bases). This can deform and inactivate the protein, and is one mechanism of heavy metal poisoning.
[edit]
Examples of thiols
- MethanethiolMethanethiolMethanethiol is a colorless gas with a smell like rotten cabbage. It is a natural substance found in the blood and brain of humans and other animal as well as plant tissues. It is disposed of through animal feces. It occurs naturally in certain foods, such as some nuts and cheese...
– CH3SH [m-mercaptan] - EthanethiolEthanethiolEthanethiol is an organic compound with the formula CH3CH2SH. It consists of an ethyl group, CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with S instead of O. The presence of S leads to many different properties, most notably the infamous odour of EtSH...
– C2H5SH [e- mercaptan] - 1-Propanethiol – C3H7SH [n-P mercaptan]
- 2-Propanethiol – CH3CH(SH)CH3 [2C3 mercaptan]
- ButanethiolButanethiolButanethiol, also known as butyl mercaptan, is a volatile, clear to yellowish liquid with a fetid odor, commonly described as "skunk" odor. In fact, butanethiol is structurally similar to several major constituents of a skunk's defensive spray...
– C4H9SH [n-butyl mercaptan] - tert-Butyl mercaptan – C(CH3)3SH [t-butyl mercaptan]
- Pentanethiols – C5H11SH [pentyl mercaptan]
- Coenzyme ACoenzyme ACoenzyme A is a coenzyme, notable for its role in the synthesis and oxidation of fatty acids, and the oxidation of pyruvate in the citric acid cycle. All sequenced genomes encode enzymes that use coenzyme A as a substrate, and around 4% of cellular enzymes use it as a substrate...
- GlutathioneGlutathioneGlutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
- CysteineCysteineCysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
- 2-Mercaptoethanol2-Mercaptoethanol2-Mercaptoethanol is the chemical compound with the formula HOCH2CH2SH. It is a hybrid of ethylene glycol, HOCH2CH2OH, and 1,2-ethanedithiol, HSCH2CH2SH...
- DithiothreitolDithiothreitolDithiothreitol is the common name for a small-molecule redox reagent known as Cleland's reagent. DTT's formula is C4H10O2S2 and the molecular structure of its reduced form is shown at the right; its oxidized form is a disulfide-bonded 6-membered ring . Its name derives from the four-carbon...
/dithioerythritolDithioerythritolDithioerythritol is a sulfur containing sugar derived from the corresponding 4-carbon monosaccharide erythrose. It is an epimer of dithiothreitol...
(an epimerEpimerIn chemistry, epimers are diastereomers that differ in configuration of only one stereogenic center. Diastereomers are a class of stereoisomers that are non-superposable, non-mirror images of one another....
ic pair) - 2-Mercaptoindole2-Mercaptoindole2-Mercaptoindole is a bicyclic heterocycle containing a thiol group. It is a popular ligand and building block for more complex structures.-References:...
- Metallothioneins
External links
- Applications, Properties, and Synthesis of w-Functionalized n-Alkanethiols and Disulfides — the Building Blocks of Self-Assembled Monolayers by D. Witt, R. Klajn, P. Barski, B.A. Grzybowski at Northwestern University.
- Mercaptan, by The Columbia Electronic Encyclopedia.
- What is Mercaptan?, by Columbia Gas of Pennsylvania and Maryland.
- What Is the Worst Smelling Chemical?, by About Chemistry.

