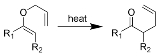
Claisen rearrangement
Encyclopedia
The Claisen rearrangement (not to be confused with the Claisen condensation
) is a powerful carbon
-carbon bond
-forming chemical reaction
discovered by Rainer Ludwig Claisen
. The heating of an allyl
vinyl
ether
will initiate a [3,3]-sigmatropic rearrangement
to give a γ,δ-unsaturated carbonyl.
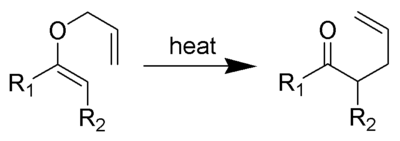 Discovered in 1912, the Claisen rearrangement is the first recorded example of a [3,3]-sigmatropic rearrangement.
Discovered in 1912, the Claisen rearrangement is the first recorded example of a [3,3]-sigmatropic rearrangement.
Many reviews have been written.
(about 84 kJ/mol), concerted pericyclic reactions which according to the Woodward-Hoffmann rules
show a suprafacial reaction pathway.
There are substantial solvent effects
in the Claisen reactions. More polar solvents tend to accelerate the reaction to a greater extent. Hydrogen-bonding solvents gave the highest rate constants. For example, ethanol
/water
solvent mixtures give rate constants 10-fold higher than sulfolane
.[1][2]
Trivalent organoaluminium
reagents, such as trimethylaluminium
, have been shown to accelerate this reaction.
of an allyl
phenyl ether
to an intermediate which quickly tautomerizes to an ortho-substituted phenol
.
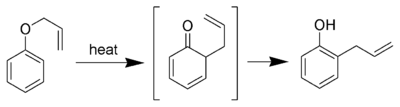 If ortho position is substituted then reaction goes to para position with retention in configration.
If ortho position is substituted then reaction goes to para position with retention in configration.
in 1964.
 Mechanism:[10]
Mechanism:[10]
ic acetate
with strong base (such as Lithium diisopropylamide
) to give a γ,δ-unsaturated carboxylic acid
.
 Mechanism:[10]
Mechanism:[10]
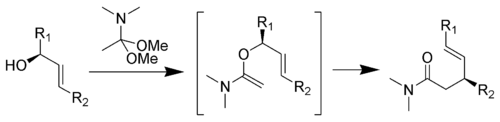
ic alcohol
with trimethyl orthoacetate to give a γ,δ-unsaturated ester
.
 Mechanism:[10]
Mechanism:[10]
can serve as one of the pi-bonded moieties in the rearrangement.

(named after Larry Overman) is a Claisen rearrangement of allylic trichloroacetimidates to allylic trichloroacetamides.
ammonium ions are highly selective for Z-enolates under mild conditions.
ion to prephenate
ion, a key intermediate in the shikimic acid
pathway (the biosynthetic
pathway towards the synthesis of phenylalanine
and tyrosine
).
Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
) is a powerful carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
-carbon bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
-forming chemical reaction
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
discovered by Rainer Ludwig Claisen
Rainer Ludwig Claisen
Rainer Ludwig Claisen was a famous German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the university of Bonn , where he became a member of K.St.V. Arminia...
. The heating of an allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
will initiate a [3,3]-sigmatropic rearrangement
Sigmatropic reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular process. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon-carbon...
to give a γ,δ-unsaturated carbonyl.

Many reviews have been written.
Mechanism
The Claisen rearrangement (and its variants) are exothermicExothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
(about 84 kJ/mol), concerted pericyclic reactions which according to the Woodward-Hoffmann rules
Woodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
show a suprafacial reaction pathway.
There are substantial solvent effects
Solvent effects
In chemistry, Solvent effects is the group of effects that a solvent has on chemical reactivity. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynamic and kinetic control over a chemical reaction.-Effects on...
in the Claisen reactions. More polar solvents tend to accelerate the reaction to a greater extent. Hydrogen-bonding solvents gave the highest rate constants. For example, ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
/water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
solvent mixtures give rate constants 10-fold higher than sulfolane
Sulfolane
Sulfolane is a clear, colorless liquid commonly used in the chemical industry as an extractive distillation solvent or reaction solvent. Sulfolane was originally developed by the Shell Oil Company in the 1960s as a solvent to purify butadiene...
.[1][2]
Trivalent organoaluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
reagents, such as trimethylaluminium
Trimethylaluminium
Trimethylaluminium is the chemical compound with the formula Al26, abbreviated as Al2Me6, 2 or the abbreviation TMA. This pyrophoric, colorless liquid is an industrially important organoaluminium compound...
, have been shown to accelerate this reaction.
Aromatic Claisen rearrangement
The aromatic variation of the Claisen rearrangement is the [3,3]-sigmatropic rearrangementSigmatropic reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular process. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon-carbon...
of an allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
phenyl ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
to an intermediate which quickly tautomerizes to an ortho-substituted phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
.

Bellus-Claisen rearrangement
The Bellus-Claisen rearrangement is the reaction of allylic ethers, amines, and thioethers with ketenes to give γ,δ-unsaturated esters, amides, and thioesters.
Eschenmoser-Claisen rearrangement
The Eschenmoser-Claisen rearrangement proceeds from an allylic alcohol to a γ,δ-unsaturated amide, and was developed by Albert EschenmoserAlbert Eschenmoser
Albert Eschenmoser is a Swiss chemist working at the ETH Zurich and The Scripps Research Institute.His work together with Lavoslav Ružička on terpenes and the postulation of squalene cyclization to form lanosterol improved the insight into steroid biosynthesis.In the early 1960s, Eschenmoser began...
in 1964.

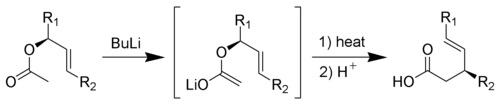
Ireland-Claisen rearrangement
The Ireland-Claisen rearrangement is the reaction of an allylAllyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
ic acetate
Acetate
An acetate is a derivative of acetic acid. This term includes salts and esters, as well as the anion found in solution. Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In...
with strong base (such as Lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
) to give a γ,δ-unsaturated carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
.

Johnson-Claisen rearrangement
The Johnson-Claisen rearrangement is the reaction of an allylAllyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
ic alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
with trimethyl orthoacetate to give a γ,δ-unsaturated ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
.

Aza-Claisen
An iminiumIminium
An iminium salt or cation in organic chemistry has the general structure [R1R2C=NR3R4]+ and is as such a protonated or substituted imine. It is an intermediate in many organic reactions such as the Beckmann rearrangement, Vilsmeier-Haack reaction, Stephen reaction or the Duff reaction...
can serve as one of the pi-bonded moieties in the rearrangement.

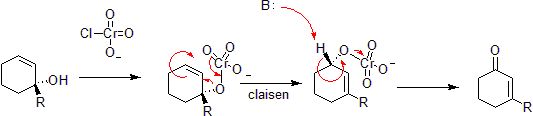
Chromium Oxidation
Chromium can oxidize allylic alcohols to alpha-beta unsaturated ketones on the opposite side of the unsaturated bond from the alcohol. This is via a concerted hetero-claisen reaction, although there are mechanistic differences since the chromium atom has access to d- shell orbitals which allow the reaction under a less constrained set of geometries.
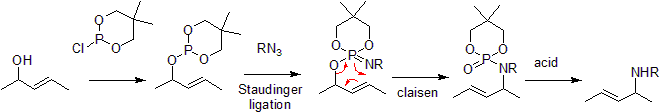
Chen-Mapp Reaction
The Chen-Mapp reaction also known as the [3,3]-Phosphorimidate Rearrangement or Staudinger-Claisen Reaction installs a phosphite in the place of an alcohol and takes advantage of the Staudinger Ligation to convert this to an imine. The subsequent claisen is driven by the fact that a P=O double bond is more energetically favorable than a P=N double bond.
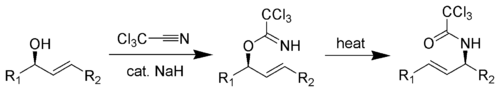
Overman rearrangement
The Overman rearrangementOverman rearrangement
The Overman rearrangement is a chemical reaction that can be described as a Claisen rearrangement of allylic alcohols to give allylic trichloroacetamides through an imidate intermediate. The Overman rearrangement was discovered in 1974 by Larry Overman....
(named after Larry Overman) is a Claisen rearrangement of allylic trichloroacetimidates to allylic trichloroacetamides.

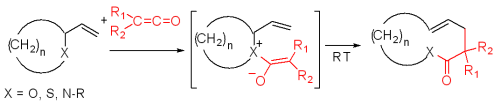
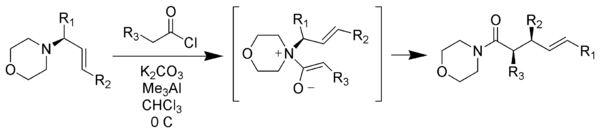
Zwitterionic Claisen rearrangement
Unlike typical Claisen rearrangements which require heating, zwitterionic Claisen rearrangements take place at or below room temperature. The acylAcyl
An acyl group is a functional group derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids.In organic chemistry, the acyl group is usually derived from a carboxylic acid . Therefore, it has the formula RCO-, where R represents an alkyl group that is...
ammonium ions are highly selective for Z-enolates under mild conditions.

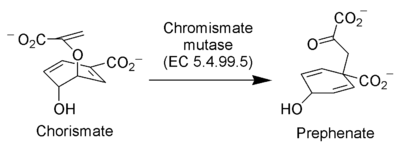
Claisen rearrangement in nature
The enzyme Chorismate mutase (EC 5.4.99.5) catalyzes the Claisen rearrangement of chorismateChorismic acid
Chorismic acid, more commonly known as its anionic form chorismate, is an important biochemical intermediate in plants and microorganisms...
ion to prephenate
Prephenic acid
Prephenic acid, commonly also known by its anionic form prephenate, is an intermediate in the biosynthesis of the aromatic amino acids phenylalanine and tyrosine.It is synthesized by a [3,3]-sigmatropic Claisen rearrangement of chorismate....
ion, a key intermediate in the shikimic acid
Shikimic acid
Shikimic acid, more commonly known as its anionic form shikimate, is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi , from which it was first isolated....
pathway (the biosynthetic
Biosynthesis
Biosynthesis is an enzyme-catalyzed process in cells of living organisms by which substrates are converted to more complex products. The biosynthesis process often consists of several enzymatic steps in which the product of one step is used as substrate in the following step...
pathway towards the synthesis of phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
and tyrosine
Tyrosine
Tyrosine or 4-hydroxyphenylalanine, is one of the 22 amino acids that are used by cells to synthesize proteins. Its codons are UAC and UAU. It is a non-essential amino acid with a polar side group...
).


