
Metabolism
Encyclopedia
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s that happen in the cells of living organism
Organism
In biology, an organism is any contiguous living system . In at least some form, all organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homoeostasis as a stable whole.An organism may either be unicellular or, as in the case of humans, comprise...
s to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories. Catabolism
Catabolism
Catabolism is the set of metabolic pathways that break down molecules into smaller units and release energy. In catabolism, large molecules such as polysaccharides, lipids, nucleic acids and proteins are broken down into smaller units such as monosaccharides, fatty acids, nucleotides, and amino...
breaks down organic matter, for example to harvest energy in cellular respiration
Cellular respiration
Cellular respiration is the set of the metabolic reactions and processes that take place in the cells of organisms to convert biochemical energy from nutrients into adenosine triphosphate , and then release waste products. The reactions involved in respiration are catabolic reactions that involve...
. Anabolism
Anabolism
Anabolism is the set of metabolic pathways that construct molecules from smaller units. These reactions require energy. One way of categorizing metabolic processes, whether at the cellular, organ or organism level is as 'anabolic' or as 'catabolic', which is the opposite...
uses energy to construct components of cells such as protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s and nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s.
The chemical reactions of metabolism are organized into metabolic pathway
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
s, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
and will not occur by themselves, by coupling
Coupling (physics)
In physics, two systems are coupled if they are interacting with each other. Of special interest is the coupling of two vibratory systems by means of springs or magnetic fields, etc...
them to spontaneous reactions
Spontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
that release energy. As enzymes act as catalysts
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
they allow these reactions to proceed quickly and efficiently. Enzymes also allow the regulation
Control theory
Control theory is an interdisciplinary branch of engineering and mathematics that deals with the behavior of dynamical systems. The desired output of a system is called the reference...
of metabolic pathways in response to changes in the cell's
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
environment or signals
Cell signaling
Cell signaling is part of a complex system of communication that governs basic cellular activities and coordinates cell actions. The ability of cells to perceive and correctly respond to their microenvironment is the basis of development, tissue repair, and immunity as well as normal tissue...
from other cells.
The metabolism of an organism determines which substances it will find nutritious
Nutrition
Nutrition is the provision, to cells and organisms, of the materials necessary to support life. Many common health problems can be prevented or alleviated with a healthy diet....
and which it will find poison
Poison
In the context of biology, poisons are substances that can cause disturbances to organisms, usually by chemical reaction or other activity on the molecular scale, when a sufficient quantity is absorbed by an organism....
ous. For example, some prokaryote
Prokaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
s use hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate
Basal metabolic rate
Basal Metabolic Rate , and the closely related resting metabolic rate , is the amount of daily energy expended by humans and other animals at rest. Rest is defined as existing in a neutrally temperate environment while in the post-absorptive state...
, influences how much food an organism will require, and also affects how it is able to obtain that food.
A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s that are best known as the intermediates in the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
are present in all known organisms, being found in species as diverse as the unicellular
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
Escherichia coli
Escherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
and huge multicellular
Multicellular organism
Multicellular organisms are organisms that consist of more than one cell, in contrast to single-celled organisms. Most life that can be seen with the the naked eye is multicellular, as are all animals and land plants.-Evolutionary history:Multicellularity has evolved independently dozens of times...
organisms like elephant
Elephant
Elephants are large land mammals in two extant genera of the family Elephantidae: Elephas and Loxodonta, with the third genus Mammuthus extinct...
s. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history
Evolutionary history of life
The evolutionary history of life on Earth traces the processes by which living and fossil organisms have evolved since life on Earth first originated until the present day. Earth formed about 4.5 Ga and life appeared on its surface within one billion years...
, and being retained because of their efficacy.
Key biochemicals
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
: amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, carbohydrate
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s and lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s (often called fat
Fat
Fats consist of a wide group of compounds that are generally soluble in organic solvents and generally insoluble in water. Chemically, fats are triglycerides, triesters of glycerol and any of several fatty acids. Fats may be either solid or liquid at room temperature, depending on their structure...
s). As these molecules are vital for life, metabolic reactions either focus on making these molecules during the construction of cells and tissues, or breaking them down and using them as a source of energy, in the digestion and use of food. Many important biochemicals can be joined together to make polymer
Polymer
A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s such as DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and proteins. These macromolecules are essential.
| Type of molecule | Name of monomer Monomer A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex... forms |
Name of polymer Polymer A polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds... forms |
Examples of polymer forms |
|---|---|---|---|
| Amino acid Amino acid Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen... s |
Amino acids | Protein Protein Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of... s (also called polypeptides) |
Fibrous protein Fibrous protein Scleroproteins, or fibrous proteins, constitute one of the three main classes of proteins, alongside globular proteins and conjugated proteins.Keratin, collagen, elastin, and fibroin are all scleroproteins... s and globular protein Globular protein Globular proteins, or spheroproteins are one of the two main protein classes, comprising "globe"-like proteins that are more or less soluble in aqueous solutions... s |
| Carbohydrate Carbohydrate A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical... s |
Monosaccharide Monosaccharide Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose... s |
Polysaccharide Polysaccharide Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,... s |
Starch Starch Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store... , glycogen Glycogen Glycogen is a molecule that serves as the secondary long-term energy storage in animal and fungal cells, with the primary energy stores being held in adipose tissue... and cellulose Cellulose Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to over ten thousand β linked D-glucose units.... |
| Nucleic acid Nucleic acid Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information... s |
Nucleotide Nucleotide Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions... s |
Polynucleotide Polynucleotide A polynucleotide molecule is a biopolymer composed of 13 or more nucleotide monomers covalently bonded in a chain. DNA and RNA are examples of polynucleotides with distinct biological function. The prefix poly comes from the ancient Greek πολυς... s |
DNA DNA Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in... and RNA RNA Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life.... |
Amino acids and proteins
ProteinProtein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s are made of amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s arranged in a linear chain and joined together by peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s. Many proteins are the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s that catalyze
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
the chemical reactions in metabolism. Other proteins have structural or mechanical functions, such as the proteins that form the cytoskeleton
Cytoskeleton
The cytoskeleton is a cellular "scaffolding" or "skeleton" contained within a cell's cytoplasm and is made out of protein. The cytoskeleton is present in all cells; it was once thought to be unique to eukaryotes, but recent research has identified the prokaryotic cytoskeleton...
, a system of scaffolding
Scaffolding
Scaffolding is a temporary structure used to support people and material in the construction or repair of buildings and other large structures. It is usually a modular system of metal pipes or tubes, although it can be from other materials...
that maintains the cell shape. Proteins are also important in cell signaling
Cell signaling
Cell signaling is part of a complex system of communication that governs basic cellular activities and coordinates cell actions. The ability of cells to perceive and correctly respond to their microenvironment is the basis of development, tissue repair, and immunity as well as normal tissue...
, immune responses
Antibody
An antibody, also known as an immunoglobulin, is a large Y-shaped protein used by the immune system to identify and neutralize foreign objects such as bacteria and viruses. The antibody recognizes a unique part of the foreign target, termed an antigen...
, cell adhesion
Cell adhesion
Cellular adhesion is the binding of a cell to a surface, extracellular matrix or another cell using cell adhesion molecules such as selectins, integrins, and cadherins. Correct cellular adhesion is essential in maintaining multicellular structure...
, active transport
Active transport
Active transport is the movement of a substance against its concentration gradient . In all cells, this is usually concerned with accumulating high concentrations of molecules that the cell needs, such as ions, glucose, and amino acids. If the process uses chemical energy, such as from adenosine...
across membranes, and the cell cycle
Cell cycle
The cell cycle, or cell-division cycle, is the series of events that takes place in a cell leading to its division and duplication . In cells without a nucleus , the cell cycle occurs via a process termed binary fission...
.
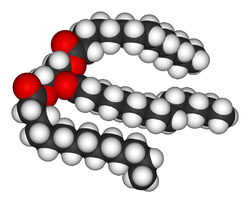
Lipids
LipidLipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s are the most diverse group of biochemicals. Their main structural uses are as part of biological membrane
Biological membrane
A biological membrane or biomembrane is an enclosing or separatingmembrane that acts as a selective barrier, within or around a cell. It consists of a lipid bilayer with embedded proteins that may constitute close to 50% of membrane content...
s such as the cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
, or as a source of energy. Lipids are usually defined as hydrophobic
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is repelled from a mass of water....
or amphipathic
Amphiphiles
Amphiphile is a term describing a chemical compound possessing both hydrophilic and lipophilic properties...
biological molecules that will dissolve in organic solvents such as benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
or chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
. The fat
Fat
Fats consist of a wide group of compounds that are generally soluble in organic solvents and generally insoluble in water. Chemically, fats are triglycerides, triesters of glycerol and any of several fatty acids. Fats may be either solid or liquid at room temperature, depending on their structure...
s are a large group of compounds that contain fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s and glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
; a glycerol molecule attached to three fatty acid ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s is a triacylglyceride
Triglyceride
A triglyceride is an ester derived from glycerol and three fatty acids. There are many triglycerides, depending on the oil source, some are highly unsaturated, some less so....
. Several variations on this basic structure exist, including alternate backbones such as sphingosine
Sphingosine
Sphingosine is an 18-carbon amino alcohol with an unsaturated hydrocarbon chain, which forms a primary part of sphingolipids, a class of cell membrane lipids that include sphingomyelin, an important phospholipid.-Functions:...
in the sphingolipid
Sphingolipid
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named for the mythological Sphinx because of their enigmatic nature. These compounds play...
s, and hydrophilic
Hydrophile
A hydrophile, from the Greek "water" and φιλια "love," is a molecule or other molecular entity that is attracted to, and tends to be dissolved by water. A hydrophilic molecule or portion of a molecule is one that has a tendency to interact with or be dissolved by, water and other polar substances...
groups such as phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
in phospholipid
Phospholipid
Phospholipids are a class of lipids that are a major component of all cell membranes as they can form lipid bilayers. Most phospholipids contain a diglyceride, a phosphate group, and a simple organic molecule such as choline; one exception to this rule is sphingomyelin, which is derived from...
s. Steroid
Steroid
A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.The core...
s such as cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
are another major class of lipids that are made in cells.
Carbohydrates
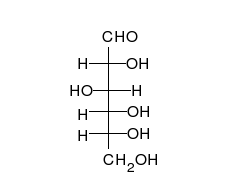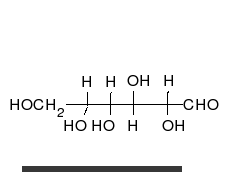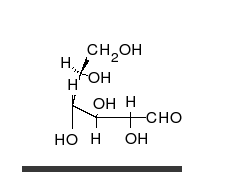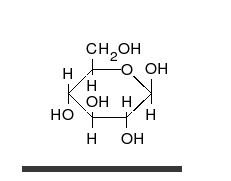
Carbohydrate
A carbohydrate is an organic compound with the empirical formula ; that is, consists only of carbon, hydrogen, and oxygen, with a hydrogen:oxygen atom ratio of 2:1 . However, there are exceptions to this. One common example would be deoxyribose, a component of DNA, which has the empirical...
s are aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
s or ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
s with many hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups that can exist as straight chains or rings. Carbohydrates are the most abundant biological molecules, and fill numerous roles, such as the storage and transport of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
(starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
, glycogen
Glycogen
Glycogen is a molecule that serves as the secondary long-term energy storage in animal and fungal cells, with the primary energy stores being held in adipose tissue...
) and structural components (cellulose
Cellulose
Cellulose is an organic compound with the formula , a polysaccharide consisting of a linear chain of several hundred to over ten thousand β linked D-glucose units....
in plants, chitin
Chitin
Chitin n is a long-chain polymer of a N-acetylglucosamine, a derivative of glucose, and is found in many places throughout the natural world...
in animals). The basic carbohydrate units are called monosaccharide
Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s and include galactose
Galactose
Galactose , sometimes abbreviated Gal, is a type of sugar that is less sweet than glucose. It is a C-4 epimer of glucose....
, fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
, and most importantly glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
. Monosaccharides can be linked together to form polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s in almost limitless ways.
Nucleotides
The two nucleic acids, DNADNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
are polymers of nucleotides, each nucleotide comprising a phosphate group, a ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
sugar group, and a nitrogenous base
Nitrogenous base
A nitrogenous base is a nitrogen-containing molecule having the chemical properties of a base. It is an organic compound that owes its property as a base to the lone pair of electrons of a nitrogen atom. In biological sciences, nitrogenous bases are typically classified as the derivatives of two...
. Nucleic acids are critical for the storage and use of genetic information, through the processes of transcription
Transcription (genetics)
Transcription is the process of creating a complementary RNA copy of a sequence of DNA. Both RNA and DNA are nucleic acids, which use base pairs of nucleotides as a complementary language that can be converted back and forth from DNA to RNA by the action of the correct enzymes...
and protein biosynthesis
Protein biosynthesis
Protein biosynthesis is the process in which cells build or manufacture proteins. The term is sometimes used to refer only to protein translation but more often it refers to a multi-step process, beginning with amino acid synthesis and transcription of nuclear DNA into messenger RNA, which is then...
. This information is protected by DNA repair
DNA repair
DNA repair refers to a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as UV light and radiation can cause DNA damage, resulting in as many as 1...
mechanisms and propagated through DNA replication
DNA replication
DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts with one double-stranded DNA molecule and produces two identical copies of the molecule...
. Many virus
Virus
A virus is a small infectious agent that can replicate only inside the living cells of organisms. Viruses infect all types of organisms, from animals and plants to bacteria and archaea...
es have an RNA genome
RNA virus
An RNA virus is a virus that has RNA as its genetic material. This nucleic acid is usually single-stranded RNA but may be double-stranded RNA...
, for example HIV
HIV
Human immunodeficiency virus is a lentivirus that causes acquired immunodeficiency syndrome , a condition in humans in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive...
, which uses reverse transcription to create a DNA template from its viral RNA genome. RNA in ribozyme
Ribozyme
A ribozyme is an RNA molecule with a well defined tertiary structure that enables it to catalyze a chemical reaction. Ribozyme means ribonucleic acid enzyme. It may also be called an RNA enzyme or catalytic RNA. Many natural ribozymes catalyze either the hydrolysis of one of their own...
s such as spliceosome
Spliceosome
A spliceosome is a complex of snRNA and protein subunits that removes introns from a transcribed pre-mRNA segment. This process is generally referred to as splicing.-Composition:...
s and ribosome
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
s is similar to enzymes as it can catalyze chemical reactions. Individual nucleoside
Nucleoside
Nucleosides are glycosylamines consisting of a nucleobase bound to a ribose or deoxyribose sugar via a beta-glycosidic linkage...
s are made by attaching a nucleobase
Nucleobase
Nucleobases are a group of nitrogen-based molecules that are required to form nucleotides, the basic building blocks of DNA and RNA. Nucleobases provide the molecular structure necessary for the hydrogen bonding of complementary DNA and RNA strands, and are key components in the formation of stable...
to a ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
sugar. These bases are heterocyclic rings containing nitrogen, classified as purines or pyrimidines. Nucleotides also act as coenzymes in metabolic group transfer reactions.
Coenzymes

Metabolism involves a vast array of chemical reactions, but most fall under a few basic types of reactions that involve the transfer of functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s. This common chemistry allows cells to use a small set of metabolic intermediates to carry chemical groups between different reactions. These group-transfer intermediates are called coenzymes. Each class of group-transfer reaction is carried out by a particular coenzyme, which is the substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
for a set of enzymes that produce it, and a set of enzymes that consume it. These coenzymes are therefore continuously being made, consumed and then recycled.
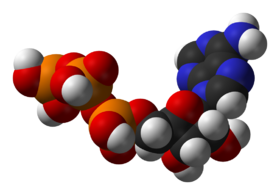
One central coenzyme is adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP), the universal energy currency of cells. This nucleotide is used to transfer chemical energy between different chemical reactions. There is only a small amount of ATP in cells, but as it is continuously regenerated, the human body can use about its own weight in ATP per day. ATP acts as a bridge between catabolism and anabolism, with catabolic reactions generating ATP and anabolic reactions consuming it. It also serves as a carrier of phosphate groups in phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
reactions.
A vitamin
Vitamin
A vitamin is an organic compound required as a nutrient in tiny amounts by an organism. In other words, an organic chemical compound is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on...
is an organic compound needed in small quantities that cannot be made in the cells. In human nutrition
Nutrition
Nutrition is the provision, to cells and organisms, of the materials necessary to support life. Many common health problems can be prevented or alleviated with a healthy diet....
, most vitamins function as coenzymes after modification; for example, all water-soluble vitamins are phosphorylated or are coupled to nucleotides when they are used in cells. Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
(NADH), a derivative of vitamin B3 (niacin
Niacin
"Niacin" redirects here. For the neo-fusion band, see Niacin .Niacin is an organic compound with the formula and, depending on the definition used, one of the forty to eighty essential human nutrients.Niacin is one of five vitamins associated with a pandemic deficiency disease: niacin deficiency...
), is an important coenzyme that acts as a hydrogen acceptor. Hundreds of separate types of dehydrogenase
Dehydrogenase
A dehydrogenase is an enzyme that oxidises a substrate by a reduction reaction that transfers one or more hydrides to an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN.-Examples:...
s remove electrons from their substrates and reduce
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
NAD+ into NADH. This reduced form of the coenzyme is then a substrate for any of the reductase
Reductase
-Examples:* 5-alpha reductase* Dihydrofolate reductase* HMG-CoA reductase* Methemoglobin reductase* Ribonucleotide reductase* Thioredoxin reductase* E. coli nitroreductase* Methylenetetrahydrofolate reductase...
s in the cell that need to reduce their substrates. Nicotinamide adenine dinucleotide exists in two related forms in the cell, NADH and NADPH. The NAD+/NADH form is more important in catabolic reactions, while NADP+/NADPH is used in anabolic reactions.
Minerals and cofactors
Inorganic elements play critical roles in metabolism; some are abundant (e.g. sodiumSodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
and potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
) while others function at minute concentrations. About 99% of a mammal's mass is made up of the elements carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
, nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
, sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
, hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, phosphorus
Phosphorus
Phosphorus is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group, phosphorus as a mineral is almost always present in its maximally oxidized state, as inorganic phosphate rocks...
, oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
. Organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s (proteins, lipids and carbohydrates) contain the majority of the carbon and nitrogen; most of the oxygen and hydrogen is present as water.
The abundant inorganic elements act as ion
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
ic electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
s. The most important ions are sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
, potassium
Potassium
Potassium is the chemical element with the symbol K and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite the hydrogen emitted in the reaction.Potassium and sodium are...
, calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
, magnesium
Magnesium
Magnesium is a chemical element with the symbol Mg, atomic number 12, and common oxidation number +2. It is an alkaline earth metal and the eighth most abundant element in the Earth's crust and ninth in the known universe as a whole...
, chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
, phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
and the organic ion bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
. The maintenance of precise gradients across cell membrane
Cell membrane
The cell membrane or plasma membrane is a biological membrane that separates the interior of all cells from the outside environment. The cell membrane is selectively permeable to ions and organic molecules and controls the movement of substances in and out of cells. It basically protects the cell...
s maintains osmotic pressure
Osmotic pressure
Osmotic pressure is the pressure which needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane....
and pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
. Ions are also critical for nerve
Nerve
A peripheral nerve, or simply nerve, is an enclosed, cable-like bundle of peripheral axons . A nerve provides a common pathway for the electrochemical nerve impulses that are transmitted along each of the axons. Nerves are found only in the peripheral nervous system...
and muscle
Muscle
Muscle is a contractile tissue of animals and is derived from the mesodermal layer of embryonic germ cells. Muscle cells contain contractile filaments that move past each other and change the size of the cell. They are classified as skeletal, cardiac, or smooth muscles. Their function is to...
function, as action potential
Action potential
In physiology, an action potential is a short-lasting event in which the electrical membrane potential of a cell rapidly rises and falls, following a consistent trajectory. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and...
s in these tissues are produced by the exchange of electrolytes between the extracellular fluid
Extracellular fluid
Extracellular fluid usually denotes all body fluid outside of cells. The remainder is called intracellular fluid.In some animals, including mammals, the extracellular fluid can be divided into two major subcompartments, interstitial fluid and blood plasma...
and the cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
. Electrolytes enter and leave cells through proteins in the cell membrane called ion channels. For example, muscle contraction
Muscle contraction
Muscle fiber generates tension through the action of actin and myosin cross-bridge cycling. While under tension, the muscle may lengthen, shorten, or remain the same...
depends upon the movement of calcium, sodium and potassium through ion channels in the cell membrane and T-tubule
T-tubule
A T-tubule is a deep invagination of the sarcolemma, which is the plasma membrane, only found in skeletal and cardiac muscle cells...
s.
Transition metal
Transition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s are usually present as trace element
Trace element
In analytical chemistry, a trace element is an element in a sample that has an average concentration of less than 100 parts per million measured in atomic count, or less than 100 micrograms per gram....
s in organisms, with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
being most abundant. These metals are used in some proteins as cofactors
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
and are essential for the activity of enzymes such as catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
and oxygen-carrier proteins such as hemoglobin
Hemoglobin
Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates, with the exception of the fish family Channichthyidae, as well as the tissues of some invertebrates...
. Metal cofactors are bound tightly to specific sites in proteins; although enzyme cofactors can be modified during catalysis, they always return to their original state by the end of the reaction catalyzed. Metal micronutrients are taken up into organisms by specific transporters and bind to storage proteins such as ferritin
Ferritin
Ferritin is a ubiquitous intracellular protein that stores iron and releases it in a controlled fashion. The amount of ferritin stored reflects the amount of iron stored. The protein is produced by almost all living organisms, including bacteria, algae and higher plants, and animals...
or metallothionein
Metallothionein
Metallothionein is a family of cysteine-rich, low molecular weight proteins. They are localized to the membrane of the Golgi apparatus...
when not being used.
Catabolism
Catabolism is the set of metabolic processes that break down large molecules. These include breaking down and oxidizing food molecules. The purpose of the catabolic reactions is to provide the energy and components needed by anabolic reactions. The exact nature of these catabolic reactions differ from organism to organism and organisms can be classified based on their sources of energy and carbon (their primary nutritional groupsPrimary nutritional groups
Primary nutritional groups are groups of organisms, divided according to the sources of energy and carbon, needed for living, growth and reproduction...
), as shown in the table below. Organic molecules are used as a source of energy by organotroph
Organotroph
An organotroph is an organism that obtains hydrogen or electrons from organic substrates . Antonym: Lithotroph- See also :* Lithotroph* Heterotroph* Primary nutritional groups...
s, while lithotroph
Lithotroph
A lithotroph is an organism that uses an inorganic substrate to obtain reducing equivalents for use in biosynthesis or energy conservation via aerobic or anaerobic respiration. Known chemolithotrophs are exclusively microbes; No known macrofauna possesses the ability to utilize inorganic...
s use inorganic substrates and phototroph
Phototroph
Phototrophs are the organisms that carry out photosynthesis to acquire energy. They use the energy from sunlight to convert carbon dioxide and water into organic material to be utilized in cellular functions such as biosynthesis and respiration.Most phototrophs are autotrophs, also known as...
s capture sunlight as chemical energy. However, all these different forms of metabolism depend on redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions that involve the transfer of electrons from reduced donor molecules such as organic molecules, water, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
, hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
or ferrous ions
Ferrous
Ferrous , in chemistry, indicates a divalent iron compound , as opposed to ferric, which indicates a trivalent iron compound ....
to acceptor molecules such as oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
or sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
. In animals these reactions involve complex organic molecules being broken down to simpler molecules, such as carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and water. In photosynthetic
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
organisms such as plants and cyanobacteria, these electron-transfer reactions do not release energy, but are used as a way of storing energy absorbed from sunlight.
| Energy source | sunlight | photo- | -troph | ||
| Preformed molecules | chemo- | ||||
| Electron donor | organic compound Organic compound An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the... |
organo- | |||
| inorganic compound Inorganic compound Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,... |
litho- | ||||
| Carbon source | organic compound Organic compound An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the... |
hetero- | |||
| inorganic compound Inorganic compound Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,... |
auto- | ||||
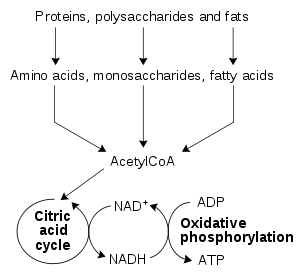
The most common set of catabolic reactions in animals can be separated into three main stages. In the first, large organic molecules such as protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s or lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s are digested into their smaller components outside cells. Next, these smaller molecules are taken up by cells and converted to yet smaller molecules, usually acetyl coenzyme A
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
(acetyl-CoA), which releases some energy. Finally, the acetyl group on the CoA is oxidised to water and carbon dioxide in the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
and electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
, releasing the energy that is stored by reducing the coenzyme nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
(NAD+) into NADH.
Digestion
Macromolecules such as starch, cellulose or proteins cannot be rapidly taken up by cells and need to be broken into their smaller units before they can be used in cell metabolism. Several common classes of enzymes digest these polymers. These digestive enzymes include proteaseProtease
A protease is any enzyme that conducts proteolysis, that is, begins protein catabolism by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain forming the protein....
s that digest proteins into amino acids, as well as glycoside hydrolase
Glycoside hydrolase
Glycoside hydrolases catalyze the hydrolysis of the glycosidic linkage to release smaller sugars...
s that digest polysaccharides into monosaccharides.
Microbes simply secrete digestive enzymes into their surroundings, while animals only secrete these enzymes from specialized cells in their guts
Gut (zoology)
In zoology, the gut, also known as the alimentary canal or alimentary tract, is a tube by which bilaterian animals transfer food to the digestion organs. In large bilaterians the gut generally also has an exit, the anus, by which the animal disposes of solid wastes...
. The amino acids or sugars released by these extracellular enzymes are then pumped into cells by specific active transport
Active transport
Active transport is the movement of a substance against its concentration gradient . In all cells, this is usually concerned with accumulating high concentrations of molecules that the cell needs, such as ions, glucose, and amino acids. If the process uses chemical energy, such as from adenosine...
proteins.
Energy from organic compounds
Carbohydrate catabolism is the breakdown of carbohydrates into smaller units. Carbohydrates are usually taken into cells once they have been digested into monosaccharideMonosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s. Once inside, the major route of breakdown is glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
, where sugars such as glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and fructose
Fructose
Fructose, or fruit sugar, is a simple monosaccharide found in many plants. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed directly into the bloodstream during digestion. Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847...
are converted into pyruvate
Pyruvic acid
Pyruvic acid is an organic acid, a ketone, as well as the simplest of the alpha-keto acids. The carboxylate ion of pyruvic acid, CH3COCOO−, is known as pyruvate, and is a key intersection in several metabolic pathways....
and some ATP is generated. Pyruvate is an intermediate in several metabolic pathways, but the majority is converted to acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
and fed into the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
. Although some more ATP is generated in the citric acid cycle, the most important product is NADH, which is made from NAD+ as the acetyl-CoA is oxidized. This oxidation releases carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
as a waste product. In anaerobic conditions, glycolysis produces lactate
Lactic acid
Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
, through the enzyme lactate dehydrogenase
Lactate dehydrogenase
Lactate dehydrogenase is an enzyme present in a wide variety of organisms, including plants and animals.Lactate dehydrogenases exist in four distinct enzyme classes. Two of them are cytochrome c-dependent enzymes, each acting on either D-lactate or L-lactate...
re-oxidizing NADH to NAD+ for re-use in glycolysis. An alternative route for glucose breakdown is the pentose phosphate pathway
Pentose phosphate pathway
The pentose phosphate pathway is a process that generates NADPH and pentoses . There are two distinct phases in the pathway. The first is the oxidative phase, in which NADPH is generated, and the second is the non-oxidative synthesis of 5-carbon sugars...
, which reduces the coenzyme NADPH and produces pentose
Pentose
A pentose is a monosaccharide with five carbon atoms. Pentoses are organized into two groups. Aldopentoses have an aldehyde functional group at position 1...
sugars such as ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
, the sugar component of nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s.
Fats are catabolised by hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
to free fatty acids and glycerol. The glycerol enters glycolysis and the fatty acids are broken down by beta oxidation
Beta oxidation
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Citric Acid cycle....
to release acetyl-CoA, which then is fed into the citric acid cycle. Fatty acids release more energy upon oxidation than carbohydrates because carbohydrates contain more oxygen in their structures.
Amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s are either used to synthesize proteins and other biomolecules, or oxidized to urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
and carbon dioxide as a source of energy. The oxidation pathway starts with the removal of the amino group by a transaminase
Transaminase
In biochemistry, a transaminase or an aminotransferase is an enzyme that catalyzes a type of reaction between an amino acid and an α-keto acid. To be specific, this reaction involves removing the amino group from the amino acid, leaving behind an α-keto acid, and transferring it to the...
. The amino group is fed into the urea cycle
Urea cycle
The urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia . This cycle was the first metabolic cycle discovered , five years before the discovery of the TCA cycle...
, leaving a deaminated carbon skeleton in the form of a keto acid
Keto acid
Keto acids are organic compounds that contain a carboxylic acid group and a ketone group. The alpha-keto acids are especially important in biology as they are involved in the Krebs citric acid cycle and in glycolysis...
. Several of these keto acids are intermediates in the citric acid cycle, for example the deamination of glutamate forms α-ketoglutarate. The glucogenic amino acid
Glucogenic amino acid
A glucogenic amino acid is an amino acid that can be converted into glucose through gluconeogenesis. This is in contrast to the ketogenic amino acids, which are converted into ketone bodies....
s can also be converted into glucose, through gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
(discussed below).
Oxidative phosphorylation
In oxidative phosphorylation, the electrons removed from organic molecules in areas such as the protagon acid cycle are transferred to oxygen and the energy released is used to make ATP. This is done in eukaryoteEukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
s by a series of proteins in the membranes of mitochondria called the electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
. In prokaryote
Prokaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
s, these proteins are found in the cell's inner membrane
Bacterial cell structure
Bacteria, despite their simplicity, contain a well-developed cell structure which is responsible for many of their unique biological properties. Many structural features are unique to bacteria and are not found among archaea or eukaryotes...
. These proteins use the energy released from passing electrons from reduced
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
molecules like NADH onto oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
to pump proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s across a membrane.
Pumping protons out of the mitochondria creates a proton concentration difference
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
across the membrane and generates an electrochemical gradient
Electrochemical gradient
An electrochemical gradient is a spatial variation of both electrical potential and chemical concentration across a membrane; that is, a combination of the membrane potential and the pH gradient...
. This force drives protons back into the mitochondrion through the base of an enzyme called ATP synthase
ATP synthase
right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms...
. The flow of protons makes the stalk subunit rotate, causing the active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
of the synthase domain to change shape and phosphorylate adenosine diphosphate
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
– turning it into ATP.
Energy from inorganic compounds
Chemolithotrophy is a type of metabolism found in prokaryoteProkaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
s where energy is obtained from the oxidation of inorganic compounds. These organisms can use hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
, reduced sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
compounds (such as sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
, hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
and thiosulfate
Thiosulfate
Thiosulfate is an oxyanion of sulfur. The prefix thio indicates that thiosulfate ion is a sulfate ion with one oxygen replaced by a sulfur. Thiosulfate occurs naturally and is produced by certain biochemical processes...
), ferrous iron (FeII)
Iron(II) oxide
Iron oxide, also known as ferrous oxide, is one of the iron oxides. It is a black-colored powder with the chemical formula . It consists of the chemical element iron in the oxidation state of 2 bonded to oxygen. Its mineral form is known as wüstite. Iron oxide should not be confused with rust,...
or ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
as sources of reducing power and they gain energy from the oxidation of these compounds with electron acceptors such as oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
or nitrite
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
. These microbial processes are important in global biogeochemical cycle
Biogeochemical cycle
In ecology and Earth science, a biogeochemical cycle or substance turnover or cycling of substances is a pathway by which a chemical element or molecule moves through both biotic and abiotic compartments of Earth. A cycle is a series of change which comes back to the starting point and which can...
s such as acetogenesis
Acetogenesis
Acetogenesis is a process through which acetate is produced by anaerobic bacteria from a variety of energy and carbon sources. The different bacterial species that are capable of acetogenesis are collectively termed acetogens.-Biochemistry:The precursor to acetic acid is the thioester acetyl CoA...
, nitrification
Nitrification
Nitrification is the biological oxidation of ammonia with oxygen into nitrite followed by the oxidation of these nitrites into nitrates. Degradation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil...
and denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
and are critical for soil fertility.
Energy from light
The energy in sunlight is captured by plants, cyanobacteria, purple bacteriaPurple bacteria
Purple bacteria or purple photosynthetic bacteria are proteobacteria that are phototrophic, that is capable of producing energy through photosynthesis...
, green sulfur bacteria
Green sulfur bacteria
The green sulfur bacteria are a family of obligately anaerobic photoautotrophic bacteria. Most closely related to the distant Bacteroidetes, they are accordingly assigned their own phylum....
and some protist
Protist
Protists are a diverse group of eukaryotic microorganisms. Historically, protists were treated as the kingdom Protista, which includes mostly unicellular organisms that do not fit into the other kingdoms, but this group is contested in modern taxonomy...
s. This process is often coupled to the conversion of carbon dioxide into organic compounds, as part of photosynthesis, which is discussed below. The energy capture and carbon fixation systems can however operate separately in prokaryotes, as purple bacteria and green sulfur bacteria can use sunlight as a source of energy, while switching between carbon fixation and the fermentation of organic compounds.
In many organisms the capture of solar energy is similar in principle to oxidative phosphorylation, as it involves energy being stored as a proton concentration gradient and this proton motive force then driving ATP synthesis. The electrons needed to drive this electron transport chain come from light-gathering proteins called photosynthetic reaction centre
Photosynthetic reaction centre
A photosynthetic reaction center is a complex of several proteins, pigments and other co-factors assembled together to execute the primary energy conversion reactions of photosynthesis...
s or rhodopsin
Rhodopsin
Rhodopsin, also known as visual purple, is a biological pigment of the retina that is responsible for both the formation of the photoreceptor cells and the first events in the perception of light. Rhodopsins belong to the G-protein coupled receptor family and are extremely sensitive to light,...
s. Reaction centers are classed into two types depending on the type of photosynthetic pigment
Photosynthetic pigment
A photosynthetic pigment is a pigment that is present in chloroplasts or photosynthetic bacteria and captures the light energy necessary for photosynthesis.- Plants :...
present, with most photosynthetic bacteria only having one type, while plants and cyanobacteria have two.
In plants, algae, and cyanobacteria, photosystem II
Photosystem
Photosystems are functional and structural units of protein complexes involved in photosynthesis that together carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons...
uses light energy to remove electrons from water, releasing oxygen as a waste product. The electrons then flow to the cytochrome b6f complex
Cytochrome b6f complex
The cytochrome b6f complex is an enzyme found in the thylakoid membrane in chloroplasts of plants, cyanobacteria, and green algae, catalyzing the transfer of electrons from plastoquinol to plastocyanin...
, which uses their energy to pump protons across the thylakoid
Thylakoid
A thylakoid is a membrane-bound compartment inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as...
membrane in the chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
. These protons move back through the membrane as they drive the ATP synthase, as before. The electrons then flow through photosystem I
Photosystem
Photosystems are functional and structural units of protein complexes involved in photosynthesis that together carry out the primary photochemistry of photosynthesis: the absorption of light and the transfer of energy and electrons...
and can then either be used to reduce the coenzyme NADP+, for use in the Calvin cycle
Calvin cycle
The Calvin cycle or Calvin–Benson-Bassham cycle or reductive pentose phosphate cycle or C3 cycle or CBB cycle is a series of biochemical redox reactions that take place in the stroma of chloroplasts in photosynthetic organisms...
which is discussed below, or recycled for further ATP generation.
Anabolism
Anabolism is the set of constructive metabolic processes where the energy released by catabolism is used to synthesize complex molecules. In general, the complex molecules that make up cellular structures are constructed step-by-step from small and simple precursors. Anabolism involves three basic stages. Firstly, the production of precursors such as amino acidAmino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s, monosaccharide
Monosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s, isoprenoids
Terpenoid
The terpenoids , sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in...
and nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
s, secondly, their activation into reactive forms using energy from ATP, and thirdly, the assembly of these precursors into complex molecules such as protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s, lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s and nucleic acid
Nucleic acid
Nucleic acids are biological molecules essential for life, and include DNA and RNA . Together with proteins, nucleic acids make up the most important macromolecules; each is found in abundance in all living things, where they function in encoding, transmitting and expressing genetic information...
s.
Organisms differ in how many of the molecules in their cells they can construct for themselves. Autotroph
Autotroph
An autotroph, or producer, is an organism that produces complex organic compounds from simple inorganic molecules using energy from light or inorganic chemical reactions . They are the producers in a food chain, such as plants on land or algae in water...
s such as plants can construct the complex organic molecules in cells such as polysaccharides and proteins from simple molecules like carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
and water. Heterotroph
Heterotroph
A heterotroph is an organism that cannot fix carbon and uses organic carbon for growth. This contrasts with autotrophs, such as plants and algae, which can use energy from sunlight or inorganic compounds to produce organic compounds such as carbohydrates, fats, and proteins from inorganic carbon...
s, on the other hand, require a source of more complex substances, such as monosaccharides and amino acids, to produce these complex molecules. Organisms can be further classified by ultimate source of their energy: photoautotrophs and photoheterotrophs obtain energy from light, whereas chemoautotrophs and chemoheterotrophs obtain energy from inorganic oxidation reactions.
Carbon fixation

Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
(CO2). In plants, cyanobacteria and algae, oxygenic photosynthesis splits water, with oxygen produced as a waste product. This process uses the ATP and NADPH produced by the photosynthetic reaction centre
Photosynthetic reaction centre
A photosynthetic reaction center is a complex of several proteins, pigments and other co-factors assembled together to execute the primary energy conversion reactions of photosynthesis...
s, as described above, to convert CO2 into glycerate 3-phosphate
Glycerate 3-phosphate
3-Phosphoglyceric acid , or glycerate 3-phosphate , is a biochemically significant 3-carbon molecule that is a metabolic intermediate in both glycolysis and the Calvin cycle. This chemical is often termed PGA when referring to the Calvin cycle...
, which can then be converted into glucose. This carbon-fixation reaction is carried out by the enzyme RuBisCO
RuBisCO
Ribulose-1,5-bisphosphate carboxylase oxygenase, commonly known by the shorter name RuBisCO, is an enzyme involved in the first major step of carbon fixation, a process by which atmospheric carbon dioxide is converted by plants to energy-rich molecules such as glucose. RuBisCo is an abbreviation...
as part of the Calvin – Benson cycle
Calvin cycle
The Calvin cycle or Calvin–Benson-Bassham cycle or reductive pentose phosphate cycle or C3 cycle or CBB cycle is a series of biochemical redox reactions that take place in the stroma of chloroplasts in photosynthetic organisms...
. Three types of photosynthesis occur in plants, C3 carbon fixation
C3 carbon fixation
carbon fixation is a metabolic pathway for carbon fixation in photosynthesis. This process converts carbon dioxide and ribulose bisphosphate into 3-phosphoglycerate through the following reaction:...
, C4 carbon fixation
C4 carbon fixation
C4 carbon fixation is one of three biochemical mechanisms, along with and CAM photosynthesis, used in carbon fixation. It is named for the 4-carbon molecule present in the first product of carbon fixation in these plants, in contrast to the 3-carbon molecule products in plants. fixation is an...
and CAM photosynthesis
Crassulacean acid metabolism
Crassulacean acid metabolism, also known as CAM photosynthesis, is a carbon fixation pathway that evolved in some plants as an adaptation to arid conditions. The stomata in the leaves remain shut during the day to reduce evapotranspiration, but open at night to collect carbon dioxide...
. These differ by the route that carbon dioxide takes to the Calvin cycle, with C3 plants fixing CO2 directly, while C4 and CAM photosynthesis incorporate the CO2 into other compounds first, as adaptations to deal with intense sunlight and dry conditions.
In photosynthetic prokaryote
Prokaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
s the mechanisms of carbon fixation are more diverse. Here, carbon dioxide can be fixed by the Calvin – Benson cycle, a reversed citric acid
Reverse Krebs cycle
The reverse Krebs cycle...
cycle, or the carboxylation of acetyl-CoA. Prokaryotic chemoautotrophs
Chemotroph
Chemotrophs are organisms that obtain energy by the oxidation of electron donors in their environments. These molecules can be organic or inorganic . The chemotroph designation is in contrast to phototrophs, which utilize solar energy...
also fix CO2 through the Calvin – Benson cycle, but use energy from inorganic compounds to drive the reaction.
Carbohydrates and glycans
In carbohydrate anabolism, simple organic acids can be converted into monosaccharideMonosaccharide
Monosaccharides are the most basic units of biologically important carbohydrates. They are the simplest form of sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose , fructose , galactose, xylose...
s such as glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and then used to assemble polysaccharide
Polysaccharide
Polysaccharides are long carbohydrate molecules, of repeated monomer units joined together by glycosidic bonds. They range in structure from linear to highly branched. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure,...
s such as starch
Starch
Starch or amylum is a carbohydrate consisting of a large number of glucose units joined together by glycosidic bonds. This polysaccharide is produced by all green plants as an energy store...
. The generation of glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
from compounds like pyruvate, lactate
Lactic acid
Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
, glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
, glycerate 3-phosphate
Glycerate 3-phosphate
3-Phosphoglyceric acid , or glycerate 3-phosphate , is a biochemically significant 3-carbon molecule that is a metabolic intermediate in both glycolysis and the Calvin cycle. This chemical is often termed PGA when referring to the Calvin cycle...
and amino acids is called gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
. Gluconeogenesis converts pyruvate to glucose-6-phosphate
Glucose-6-phosphate
Glucose 6-phosphate is glucose sugar phosphorylated on carbon 6. This compound is very common in cells as the vast majority of glucose entering a cell will become phosphorylated in this way....
through a series of intermediates, many of which are shared with glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
. However, this pathway is not simply glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
run in reverse, as several steps are catalyzed by non-glycolytic enzymes. This is important as it allows the formation and breakdown of glucose to be regulated separately and prevents both pathways from running simultaneously in a futile cycle
Futile cycle
A futile cycle, also known as a substrate cycle, occurs when two metabolic pathways run simultaneously in opposite directions and have no overall effect other than to dissipate energy in the form of heat...
.
Although fat is a common way of storing energy, in vertebrate
Vertebrate
Vertebrates are animals that are members of the subphylum Vertebrata . Vertebrates are the largest group of chordates, with currently about 58,000 species described. Vertebrates include the jawless fishes, bony fishes, sharks and rays, amphibians, reptiles, mammals, and birds...
s such as humans the fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s in these stores cannot be converted to glucose through gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
as these organisms cannot convert acetyl-CoA into pyruvate; plants do, but animals do not, have the necessary enzymatic machinery. As a result, after long-term starvation, vertebrates need to produce ketone bodies
Ketone bodies
Ketone bodies are three water-soluble compounds that are produced as by-products when fatty acids are broken down for energy in the liver and kidney. They are used as a source of energy in the heart and brain. In the brain, they are a vital source of energy during fasting...
from fatty acids to replace glucose in tissues such as the brain that cannot metabolize fatty acids. In other organisms such as plants and bacteria, this metabolic problem is solved using the glyoxylate cycle
Glyoxylate cycle
The glyoxylate cycle, a variation of the Tricarboxylic Acid Cycle, is an anabolic metabolic pathway occurring in plants, bacteria, protists, fungi and several microorganisms, such as E. coli and yeast. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of...
, which bypasses the decarboxylation
Decarboxylation
Decarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
step in the citric acid cycle and allows the transformation of acetyl-CoA to oxaloacetate, where it can be used for the production of glucose.
Polysaccharides and glycans
Glycans
The term glycan refers to a polysaccharide or oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan composed of beta-1,4-linked D-glucose, and chitin is a glycan composed of beta-1,4-linked N-acetyl-D-glucosamine...
are made by the sequential addition of monosaccharides by glycosyltransferase
Glycosyltransferase
Glycosyltransferases are enzymes that act as a catalyst for the transfer of a monosaccharide unit from an activated nucleotide sugar to a glycosyl acceptor molecule, usually an alcohol....
from a reactive sugar-phosphate donor such as uridine diphosphate glucose
Uridine diphosphate glucose
Uridine diphosphate glucose is a nucleotide sugar. It is involved in glycosyltransferase reactions in metabolism.-Functions:...
(UDP-glucose) to an acceptor hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group on the growing polysaccharide. As any of the hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
groups on the ring of the substrate can be acceptors, the polysaccharides produced can have straight or branched structures. The polysaccharides produced can have structural or metabolic functions themselves, or be transferred to lipids and proteins by enzymes called oligosaccharyltransferase
Oligosaccharyltransferase
Oligosaccharyltransferase or OST is a membrane protein complex that transfers a 14-sugar oligosaccharide from dolichol to nascent protein. It is a type of glycosyltransferase...
s.
Fatty acids, isoprenoids and steroids
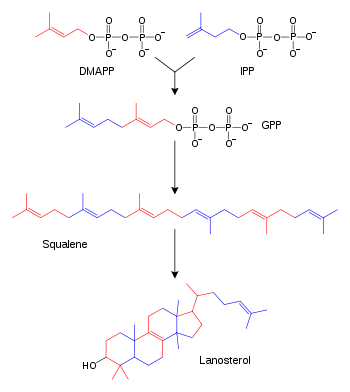
Fatty acid synthase
Fatty acid synthase is an enzyme that in humans is encoded by the FASN gene.Fatty acid synthase is a multi-enzyme protein that catalyzes fatty acid synthesis...
s that polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the actyl group, reduce it to an alcohol, dehydrate
Dehydration reaction
In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions...
it to an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
group and then reduce it again to an alkane
Alkane
Alkanes are chemical compounds that consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds without any cycles...
group. The enzymes of fatty acid biosynthesis are divided into two groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional type I protein, while in plant plastid
Plastid
Plastids are major organelles found in the cells of plants and algae. Plastids are the site of manufacture and storage of important chemical compounds used by the cell...
s and bacteria separate type II enzymes perform each step in the pathway.
Terpene
Terpene
Terpenes are a large and diverse class of organic compounds, produced by a variety of plants, particularly conifers, though also by some insects such as termites or swallowtail butterflies, which emit terpenes from their osmeterium. They are often strong smelling and thus may have had a protective...
s and isoprenoids
Terpenoid
The terpenoids , sometimes called isoprenoids, are a large and diverse class of naturally occurring organic chemicals similar to terpenes, derived from five-carbon isoprene units assembled and modified in thousands of ways. Most are multicyclic structures that differ from one another not only in...
are a large class of lipids that include the carotenoid
Carotenoid
Carotenoids are tetraterpenoid organic pigments that are naturally occurring in the chloroplasts and chromoplasts of plants and some other photosynthetic organisms like algae, some bacteria, and some types of fungus. Carotenoids can be synthesized fats and other basic organic metabolic building...
s and form the largest class of plant natural product
Natural product
A natural product is a chemical compound or substance produced by a living organism - found in nature that usually has a pharmacological or biological activity for use in pharmaceutical drug discovery and drug design...
s. These compounds are made by the assembly and modification of isoprene
Isoprene
Isoprene , or 2-methyl-1,3-butadiene, is a common organic compound with the formula CH2=CCH=CH2. Under standard conditions it is a colorless liquid...
units donated from the reactive precursors isopentenyl pyrophosphate
Isopentenyl pyrophosphate
Isopentenyl pyrophosphate is an intermediate in the classical, HMG-CoA reductase pathway used by organisms in the biosynthesis of terpenes and terpenoids. IPP is formed from acetyl-CoA via mevalonic acid...
and dimethylallyl pyrophosphate
Dimethylallyl pyrophosphate
Dimethylallyl pyrophosphate is an intermediate product of both mevalonic acid pathway and DOXP/MEP pathway. It is an isomer of isopentenyl pyrophosphate and exists in virtually all life forms...
. These precursors can be made in different ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA, while in plants and bacteria the non-mevalonate pathway
Non-mevalonate pathway
The non-mevalonate pathway or 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate pathway of isoprenoid biosynthesis is an alternative metabolic pathway leading to the formation of isopentenyl pyrophosphate and dimethylallyl pyrophosphate that has been elucidated only...
uses pyruvate and glyceraldehyde 3-phosphate
Glyceraldehyde 3-phosphate
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GADP, GAP, TP, GALP or PGAL, is a chemical compound that occurs as an intermediate in several central metabolic pathways of all organisms...
as substrates. One important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to make squalene
Squalene
Squalene is a natural organic compound originally obtained for commercial purposes primarily from shark liver oil, though plant sources are used as well, including amaranth seed, rice bran, wheat germ, and olives. All plants and animals produce squalene, including humans...
and then folded up and formed into a set of rings to make lanosterol
Lanosterol
Lanosterol is a tetracyclic triterpenoid, which is the compound from which all steroids are derived.-Role in creation of steroids:Elaboration of lanosterol under enzyme catalysis leads to the core structure of steroids. 14-Demethylation of lanosterol by CYP51 eventually yields...
. Lanosterol can then be converted into other steroids such as cholesterol
Cholesterol
Cholesterol is a complex isoprenoid. Specifically, it is a waxy steroid of fat that is produced in the liver or intestines. It is used to produce hormones and cell membranes and is transported in the blood plasma of all mammals. It is an essential structural component of mammalian cell membranes...
and ergosterol
Ergosterol
Ergosterol is a sterol found in fungi, and named for ergot, a common name for the members of the fungal genus Claviceps from which ergosterol was first isolated. Ergosterol does not occur in plant or animal cells...
.
Proteins
Organisms vary in their ability to synthesize the 20 common amino acids. Most bacteria and plants can synthesize all twenty, but mammals can synthesize only eleven nonessential amino acids. Thus, nine essential amino acidEssential amino acid
An essential amino acid or indispensable amino acid is an amino acid that cannot be synthesized de novo by the organism , and therefore must be supplied in the diet.-Essentiality vs. conditional essentiality in humans:...
s must be obtained from food. All amino acids are synthesized from intermediates in glycolysis, the citric acid cycle, or the pentose phosphate pathway. Nitrogen is provided by glutamate and glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
. Amino acid synthesis depends on the formation of the appropriate alpha-keto acid, which is then transaminated
Transaminase
In biochemistry, a transaminase or an aminotransferase is an enzyme that catalyzes a type of reaction between an amino acid and an α-keto acid. To be specific, this reaction involves removing the amino group from the amino acid, leaving behind an α-keto acid, and transferring it to the...
to form an amino acid.
Amino acids are made into proteins by being joined together in a chain by peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s. Each different protein has a unique sequence of amino acid residues: this is its primary structure
Primary structure
The primary structure of peptides and proteins refers to the linear sequence of its amino acid structural units. The term "primary structure" was first coined by Linderstrøm-Lang in 1951...
. Just as the letters of the alphabet can be combined to form an almost endless variety of words, amino acids can be linked in varying sequences to form a huge variety of proteins. Proteins are made from amino acids that have been activated by attachment to a transfer RNA
Transfer RNA
Transfer RNA is an adaptor molecule composed of RNA, typically 73 to 93 nucleotides in length, that is used in biology to bridge the three-letter genetic code in messenger RNA with the twenty-letter code of amino acids in proteins. The role of tRNA as an adaptor is best understood by...
molecule through an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
bond. This aminoacyl-tRNA precursor is produced in an ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
-dependent reaction carried out by an aminoacyl tRNA synthetase
Aminoacyl tRNA synthetase
An aminoacyl tRNA synthetase is an enzyme that catalyzes the esterification of a specific amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA. This is sometimes called "charging" the tRNA with the amino acid...
. This aminoacyl-tRNA is then a substrate for the ribosome
Ribosome
A ribosome is a component of cells that assembles the twenty specific amino acid molecules to form the particular protein molecule determined by the nucleotide sequence of an RNA molecule....
, which joins the amino acid onto the elongating protein chain, using the sequence information in a messenger RNA
Messenger RNA
Messenger RNA is a molecule of RNA encoding a chemical "blueprint" for a protein product. mRNA is transcribed from a DNA template, and carries coding information to the sites of protein synthesis: the ribosomes. Here, the nucleic acid polymer is translated into a polymer of amino acids: a protein...
.
Nucleotide synthesis and salvage
Nucleotides are made from amino acids, carbon dioxide and formic acidFormic acid
Formic acid is the simplest carboxylic acid. Its chemical formula is HCOOH or HCO2H. It is an important intermediate in chemical synthesis and occurs naturally, most notably in the venom of bee and ant stings. In fact, its name comes from the Latin word for ant, formica, referring to its early...
in pathways that require large amounts of metabolic energy. Consequently, most organisms have efficient systems to salvage preformed nucleotides. Purine
Purine
A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature....
s are synthesized as nucleoside
Nucleoside
Nucleosides are glycosylamines consisting of a nucleobase bound to a ribose or deoxyribose sugar via a beta-glycosidic linkage...
s (bases attached to ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
). Both adenine
Adenine
Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
and guanine
Guanine
Guanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine . In DNA, guanine is paired with cytosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with...
are made from the precursor nucleoside inosine
Inosine
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring via a β-N9-glycosidic bond....
monophosphate, which is synthesized using atoms from the amino acids glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
, glutamine
Glutamine
Glutamine is one of the 20 amino acids encoded by the standard genetic code. It is not recognized as an essential amino acid but may become conditionally essential in certain situations, including intensive athletic training or certain gastrointestinal disorders...
, and aspartic acid
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
, as well as formate
Formate
Formate or methanoate is the ion CHOO− or HCOO− . It is the simplest carboxylate anion. It is produced in large amounts in the hepatic mitochondria of embryonic cells and in cancer cells by the folate cycle Formate or methanoate is the ion CHOO− or HCOO− (formic acid minus one hydrogen ion). It...
transferred from the coenzyme tetrahydrofolate
Folic acid
Folic acid and folate , as well as pteroyl-L-glutamic acid, pteroyl-L-glutamate, and pteroylmonoglutamic acid are forms of the water-soluble vitamin B9...
. Pyrimidine
Pyrimidine
Pyrimidine is a heterocyclic aromatic organic compound similar to benzene and pyridine, containing two nitrogen atoms at positions 1 and 3 of the six-member ring...
s, on the other hand, are synthesized from the base orotate
Pyrimidinecarboxylic acid
Orotic acid is a heterocyclic compound and an acid; it is also known as pyrimidinecarboxylic acid. Historically it was believed to be part of the Vitamin B complex and was called vitamin B13, but it is now known that it is not a vitamin....
, which is formed from glutamine and aspartate.
Xenobiotics and redox metabolism
All organisms are constantly exposed to compounds that they cannot use as foods and would be harmful if they accumulated in cells, as they have no metabolic function. These potentially damaging compounds are called xenobioticXenobiotic
A xenobiotic is a chemical which is found in an organism but which is not normally produced or expected to be present in it. It can also cover substances which are present in much higher concentrations than are usual...
s. Xenobiotics such as synthetic drugs
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
, natural poisons
Poison
In the context of biology, poisons are substances that can cause disturbances to organisms, usually by chemical reaction or other activity on the molecular scale, when a sufficient quantity is absorbed by an organism....
and antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
s are detoxified by a set of xenobiotic-metabolizing enzymes. In humans, these include cytochrome P450 oxidases, UDP-glucuronosyltransferases
Glucuronosyltransferase
Uridine 5'-diphospho-glucuronosyltransferase is a glycosyltransferase that catalyzes addition of the glycosyl group from a UTP-sugar to a small hydrophobic molecule.This is glucuronidation reaction.Alternative names:...
, and glutathione S-transferases
Glutathione S-transferase
Enzymes of the glutathione S-transferase family are composed of many cytosolic, mitochondrial, and microsomal proteins. GSTs are present in eukaryotes and in prokaryotes, where they catalyze a variety of reactions and accept endogenous and xenobiotic substrates.GSTs can constitute up to 10% of...
. This system of enzymes acts in three stages to firstly oxidize the xenobiotic (phase I) and then conjugate water-soluble groups onto the molecule (phase II). The modified water-soluble xenobiotic can then be pumped out of cells and in multicellular organisms may be further metabolized before being excreted (phase III). In ecology
Ecology
Ecology is the scientific study of the relations that living organisms have with respect to each other and their natural environment. Variables of interest to ecologists include the composition, distribution, amount , number, and changing states of organisms within and among ecosystems...
, these reactions are particularly important in microbial biodegradation
Biodegradation
Biodegradation or biotic degradation or biotic decomposition is the chemical dissolution of materials by bacteria or other biological means...
of pollutants and the bioremediation
Bioremediation
Bioremediation is the use of microorganism metabolism to remove pollutants. Technologies can be generally classified as in situ or ex situ. In situ bioremediation involves treating the contaminated material at the site, while ex situ involves the removal of the contaminated material to be treated...
of contaminated land and oil spills. Many of these microbial reactions are shared with multicellular organisms, but due to the incredible diversity of types of microbes these organisms are able to deal with a far wider range of xenobiotics than multicellular organisms, and can degrade even persistent organic pollutant
Persistent organic pollutant
thumb|right|275px|State parties to the Stockholm Convention on Persistent Organic PollutantsPersistent organic pollutants are organic compounds that are resistant to environmental degradation through chemical, biological, and photolytic processes...
s such as organochloride
Organochloride
An organochloride, organochlorine, chlorocarbon, chlorinated hydrocarbon, or chlorinated solvent is an organic compound containing at least one covalently bonded chlorine atom. Their wide structural variety and divergent chemical properties lead to a broad range of applications...
compounds.
A related problem for aerobic organism
Aerobic organism
An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment.Faculitative anaerobes grow and survive in an oxygenated environment and so do aerotolerant anaerobes.-Glucose:...
s is oxidative stress
Oxidative stress
Oxidative stress represents an imbalance between the production and manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage...
. Here, processes including oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
and the formation of disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s during protein folding
Protein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
produce reactive oxygen species
Reactive oxygen species
Reactive oxygen species are chemically reactive molecules containing oxygen. Examples include oxygen ions and peroxides. Reactive oxygen species are highly reactive due to the presence of unpaired valence shell electrons....
such as hydrogen peroxide
Hydrogen peroxide
Hydrogen peroxide is the simplest peroxide and an oxidizer. Hydrogen peroxide is a clear liquid, slightly more viscous than water. In dilute solution, it appears colorless. With its oxidizing properties, hydrogen peroxide is often used as a bleach or cleaning agent...
. These damaging oxidants are removed by antioxidant
Antioxidant
An antioxidant is a molecule capable of inhibiting the oxidation of other molecules. Oxidation is a chemical reaction that transfers electrons or hydrogen from a substance to an oxidizing agent. Oxidation reactions can produce free radicals. In turn, these radicals can start chain reactions. When...
metabolites such as glutathione
Glutathione
Glutathione is a tripeptide that contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side-chain...
and enzymes such as catalase
Catalase
Catalase is a common enzyme found in nearly all living organisms that are exposed to oxygen, where it catalyzes the decomposition of hydrogen peroxide to water and oxygen...
s and peroxidase
Peroxidase
Peroxidases are a large family of enzymes that typically catalyze a reaction of the form:For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides...
s.
Thermodynamics of living organisms
Living organisms must obey the laws of thermodynamicsLaws of thermodynamics
The four laws of thermodynamics summarize its most important facts. They define fundamental physical quantities, such as temperature, energy, and entropy, in order to describe thermodynamic systems. They also describe the transfer of energy as heat and work in thermodynamic processes...
, which describe the transfer of heat and work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
. The second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
states that in any closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
, the amount of entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
(disorder) will tend to increase. Although living organisms' amazing complexity appears to contradict this law, life is possible as all organisms are open systems that exchange matter and energy with their surroundings. Thus living systems are not in equilibrium
Thermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
, but instead are dissipative system
Dissipative system
A dissipative system is a thermodynamically open system which is operating out of, and often far from, thermodynamic equilibrium in an environment with which it exchanges energy and matter....
s that maintain their state of high complexity by causing a larger increase in the entropy of their environments. The metabolism of a cell achieves this by coupling the spontaneous process
Spontaneous process
A spontaneous process is the time-evolution of a system in which it releases free energy and moves to a lower, more thermodynamically stable energy state...
es of catabolism to the non-spontaneous processes of anabolism. In thermodynamic
Non-equilibrium thermodynamics
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with systems that are not in thermodynamic equilibrium. Most systems found in nature are not in thermodynamic equilibrium; for they are changing or can be triggered to change over time, and are continuously and discontinuously...
terms, metabolism maintains order by creating disorder.
Regulation and control
As the environments of most organisms are constantly changing, the reactions of metabolism must be finely regulatedControl theory
Control theory is an interdisciplinary branch of engineering and mathematics that deals with the behavior of dynamical systems. The desired output of a system is called the reference...
to maintain a constant set of conditions within cells, a condition called homeostasis
Homeostasis
Homeostasis is the property of a system that regulates its internal environment and tends to maintain a stable, constant condition of properties like temperature or pH...
. Metabolic regulation also allows organisms to respond to signals and interact actively with their environments. Two closely linked concepts are important for understanding how metabolic pathways are controlled. Firstly, the regulation of an enzyme in a pathway is how its activity is increased and decreased in response to signals. Secondly, the control exerted by this enzyme is the effect that these changes in its activity have on the overall rate of the pathway (the flux
Flux
In the various subfields of physics, there exist two common usages of the term flux, both with rigorous mathematical frameworks.* In the study of transport phenomena , flux is defined as flow per unit area, where flow is the movement of some quantity per time...
through the pathway). For example, an enzyme may show large changes in activity (i.e. it is highly regulated) but if these changes have little effect on the flux of a metabolic pathway, then this enzyme is not involved in the control of the pathway.
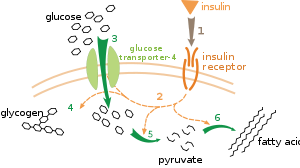
Flux
In the various subfields of physics, there exist two common usages of the term flux, both with rigorous mathematical frameworks.* In the study of transport phenomena , flux is defined as flow per unit area, where flow is the movement of some quantity per time...
through the pathway to compensate. This type of regulation often involves allosteric regulation
Allosteric regulation
In biochemistry, allosteric regulation is the regulation of an enzyme or other protein by binding an effector molecule at the protein's allosteric site . Effectors that enhance the protein's activity are referred to as allosteric activators, whereas those that decrease the protein's activity are...
of the activities of multiple enzymes in the pathway. Extrinsic control involves a cell in a multicellular organism changing its metabolism in response to signals from other cells. These signals are usually in the form of soluble messengers such as hormone
Hormone
A hormone is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. In essence, it is a chemical messenger that transports a signal from one...
s and growth factor
Growth factor
A growth factor is a naturally occurring substance capable of stimulating cellular growth, proliferation and cellular differentiation. Usually it is a protein or a steroid hormone. Growth factors are important for regulating a variety of cellular processes....
s and are detected by specific receptors
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
on the cell surface. These signals are then transmitted inside the cell by second messenger system
Second messenger system
Second messengers are molecules that relay signals from receptors on the cell surface to target molecules inside the cell, in the cytoplasm or nucleus. They relay the signals of hormones like epinephrine , growth factors, and others, and cause some kind of change in the activity of the cell...
s that often involved the phosphorylation
Phosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
of proteins.
A very well understood example of extrinsic control is the regulation of glucose metabolism by the hormone insulin
Insulin
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
. Insulin is produced in response to rises in blood glucose levels
Blood sugar
The blood sugar concentration or blood glucose level is the amount of glucose present in the blood of a human or animal. Normally in mammals, the body maintains the blood glucose level at a reference range between about 3.6 and 5.8 mM , or 64.8 and 104.4 mg/dL...
. Binding of the hormone to insulin receptor
Insulin receptor
In molecular biology, the insulin receptor is a transmembrane receptor that is activated by insulin. It belongs to the large class of tyrosine kinase receptors....
s on cells then activates a cascade of protein kinase
Protein kinase
A protein kinase is a kinase enzyme that modifies other proteins by chemically adding phosphate groups to them . Phosphorylation usually results in a functional change of the target protein by changing enzyme activity, cellular location, or association with other proteins...
s that cause the cells to take up glucose and convert it into storage molecules such as fatty acids and glycogen
Glycogen
Glycogen is a molecule that serves as the secondary long-term energy storage in animal and fungal cells, with the primary energy stores being held in adipose tissue...
. The metabolism of glycogen is controlled by activity of phosphorylase
Phosphorylase
Phosphorylases are enzymes that catalyze the addition of a phosphate group from an inorganic phosphate to an acceptor.They include allosteric enzymes that catalyze the production of glucose-1-phosphate from a glucan such as glycogen, starch or maltodextrin. Phosphorylase is also a common name used...
, the enzyme that breaks down glycogen, and glycogen synthase
Glycogen synthase
Glycogen synthase is an enzyme involved in converting glucose to glycogen. It takes short polymers of glucose and converts them into long polymers....
, the enzyme that makes it. These enzymes are regulated in a reciprocal fashion, with phosphorylation inhibiting glycogen synthase, but activating phosphorylase. Insulin causes glycogen synthesis by activating protein phosphatases
Phosphatase
A phosphatase is an enzyme that removes a phosphate group from its substrate by hydrolysing phosphoric acid monoesters into a phosphate ion and a molecule with a free hydroxyl group . This action is directly opposite to that of phosphorylases and kinases, which attach phosphate groups to their...
and producing a decrease in the phosphorylation of these enzymes.
Evolution

Three-domain system
The three-domain system is a biological classification introduced by Carl Woese in 1977 that divides cellular life forms into archaea, bacteria, and eukaryote domains. In particular, it emphasizes the separation of prokaryotes into two groups, originally called Eubacteria and Archaebacteria...
of living things and were present in the last universal ancestor
Last universal ancestor
The last universal ancestor , also called the last universal common ancestor , or the cenancestor, is the most recent organism from which all organisms now living on Earth descend. Thus it is the most recent common ancestor of all current life on Earth...
. This universal ancestral cell was prokaryotic
Prokaryote
The prokaryotes are a group of organisms that lack a cell nucleus , or any other membrane-bound organelles. The organisms that have a cell nucleus are called eukaryotes. Most prokaryotes are unicellular, but a few such as myxobacteria have multicellular stages in their life cycles...
and probably a methanogen
Methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in anoxic conditions. They are classified as archaea, a group quite distinct from bacteria...
that had extensive amino acid, nucleotide, carbohydrate and lipid metabolism. The retention of these ancient pathways during later evolution
Evolution
Evolution is any change across successive generations in the heritable characteristics of biological populations. Evolutionary processes give rise to diversity at every level of biological organisation, including species, individual organisms and molecules such as DNA and proteins.Life on Earth...
may be the result of these reactions being an optimal solution to their particular metabolic problems, with pathways such as glycolysis and the citric acid cycle producing their end products highly efficiently and in a minimal number of steps. Mutation changes that affect non-coding DNA segments may merely affect the metabolic efficiency of the individual for whom the mutation occurs.
The first pathways of enzyme-based metabolism may have been parts of purine
Purine
A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring. Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen-containing heterocycle in nature....
nucleotide metabolism, with previous metabolic pathways being part of the ancient RNA world
RNA world hypothesis
The RNA world hypothesis proposes that life based on ribonucleic acid pre-dates the current world of life based on deoxyribonucleic acid , RNA and proteins. RNA is able both to store genetic information, like DNA, and to catalyze chemical reactions, like an enzyme protein...
.
Many models have been proposed to describe the mechanisms by which novel metabolic pathways evolve. These include the sequential addition of novel enzymes to a short ancestral pathway, the duplication and then divergence of entire pathways as well as the recruitment of pre-existing enzymes and their assembly into a novel reaction pathway. The relative importance of these mechanisms is unclear, but genomic studies have shown that enzymes in a pathway are likely to have a shared ancestry, suggesting that many pathways have evolved in a step-by-step fashion with novel functions being created from pre-existing steps in the pathway. An alternative model comes from studies that trace the evolution of proteins' structures in metabolic networks, this has suggested that enzymes are pervasively recruited, borrowing enzymes to perform similar functions in different metabolic pathways (evident in the MANET database
MANET database
The Molecular Ancestry Network database is a bioinformatics database that maps evolutionary relationships of protein architectures directly onto biological networks. It was originally developed by Hee Shin Kim, Jay E...
) These recruitment processes result in an evolutionary enzymatic mosaic. A third possibility is that some parts of metabolism might exist as "modules" that can be reused in different pathways and perform similar functions on different molecules.
As well as the evolution of new metabolic pathways, evolution can also cause the loss of metabolic functions. For example, in some parasites metabolic processes that are not essential for survival are lost and preformed amino acids, nucleotides and carbohydrates may instead be scavenged from the host
Host (biology)
In biology, a host is an organism that harbors a parasite, or a mutual or commensal symbiont, typically providing nourishment and shelter. In botany, a host plant is one that supplies food resources and substrate for certain insects or other fauna...
. Similar reduced metabolic capabilities are seen in endosymbiotic
Endosymbiont
An endosymbiont is any organism that lives within the body or cells of another organism, i.e. forming an endosymbiosis...
organisms.
Investigation and manipulation

Reductionism
Reductionism can mean either an approach to understanding the nature of complex things by reducing them to the interactions of their parts, or to simpler or more fundamental things or a philosophical position that a complex system is nothing but the sum of its parts, and that an account of it can...
approach that focuses on a single metabolic pathway. Particularly valuable is the use of radioactive tracer
Radioactive tracer
A radioactive tracer, also called a radioactive label, is a substance containing a radioisotope that is used to measure the speed of chemical processes and to track the movement of a substance through a natural system such as a cell or tissue...
s at the whole-organism, tissue and cellular levels, which define the paths from precursors to final products by identifying radioactively labelled intermediates and products. The enzymes that catalyze these chemical reactions can then be purified
Protein purification
Protein purification is a series of processes intended to isolate a single type of protein from a complex mixture. Protein purification is vital for the characterization of the function, structure and interactions of the protein of interest. The starting material is usually a biological tissue or...
and their kinetics
Enzyme kinetics
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated...
and responses to inhibitors
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
investigated. A parallel approach is to identify the small molecules in a cell or tissue; the complete set of these molecules is called the metabolome
Metabolome
Metabolome refers to the complete set of small-molecule metabolites to be found within a biological sample, such as a single organism...
. Overall, these studies give a good view of the structure and function of simple metabolic pathways, but are inadequate when applied to more complex systems such as the metabolism of a complete cell.
An idea of the complexity of the metabolic network
Metabolic network
A metabolic network is the complete set of metabolic and physical processes that determine the physiological and biochemical properties of a cell...
s in cells that contain thousands of different enzymes is given by the figure showing the interactions between just 43 proteins and 40 metabolites to the right: the sequences of genomes provide lists containing anything up to 45,000 genes. However, it is now possible to use this genomic data to reconstruct complete networks of biochemical reactions and produce more holistic
Holism
Holism is the idea that all the properties of a given system cannot be determined or explained by its component parts alone...
mathematical models that may explain and predict their behavior. These models are especially powerful when used to integrate the pathway and metabolite data obtained through classical methods with data on gene expression
Gene expression
Gene expression is the process by which information from a gene is used in the synthesis of a functional gene product. These products are often proteins, but in non-protein coding genes such as ribosomal RNA , transfer RNA or small nuclear RNA genes, the product is a functional RNA...
from proteomic
Proteomics
Proteomics is the large-scale study of proteins, particularly their structures and functions. Proteins are vital parts of living organisms, as they are the main components of the physiological metabolic pathways of cells. The term "proteomics" was first coined in 1997 to make an analogy with...
and DNA microarray
DNA microarray
A DNA microarray is a collection of microscopic DNA spots attached to a solid surface. Scientists use DNA microarrays to measure the expression levels of large numbers of genes simultaneously or to genotype multiple regions of a genome...
studies. Using these techniques, a model of human metabolism has now been produced, which will guide future drug discovery and biochemical research. These models are now being used in network analysis
Network theory
Network theory is an area of computer science and network science and part of graph theory. It has application in many disciplines including statistical physics, particle physics, computer science, biology, economics, operations research, and sociology...
, to classify human diseases into groups that share common proteins or metabolites.
Bacterial metabolic networks seem to be a striking example of bow-tie
Bow tie (biology)
The term bow tie refers in science to a recent concept that tries to grasp the essence of some operational and functional structures observed in biological organisms and other kinds of complex and self-organizing systems...
organization, an architecture able to input a wide range of nutrients and produce a large variety of products and complex macromolecules using a relatively few intermediate common currencies.
A major technological application of this information is metabolic engineering
Metabolic engineering
Metabolic engineering is the practice of optimizing genetic and regulatory processes within cells to increase the cells' production of a certain substance. These processes are chemical networks that use a series of biochemical reactions and enzymes that allow cells to convert raw materials into...
. Here, organisms such as yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
, plants or bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
are genetically modified to make them more useful in biotechnology
Biotechnology
Biotechnology is a field of applied biology that involves the use of living organisms and bioprocesses in engineering, technology, medicine and other fields requiring bioproducts. Biotechnology also utilizes these products for manufacturing purpose...
and aid the production of drug
Drug
A drug, broadly speaking, is any substance that, when absorbed into the body of a living organism, alters normal bodily function. There is no single, precise definition, as there are different meanings in drug control law, government regulations, medicine, and colloquial usage.In pharmacology, a...
s such as antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
s or industrial chemicals such as 1,3-propanediol
1,3-Propanediol
1,3-Propanediol is the organic compound with the formula CH22. This three-carbon diol is a colorless viscous liquid that is miscible with water.-Products:...
and shikimic acid
Shikimic acid
Shikimic acid, more commonly known as its anionic form shikimate, is an important biochemical metabolite in plants and microorganisms. Its name comes from the Japanese flower shikimi , from which it was first isolated....
. These genetic modifications usually aim to reduce the amount of energy used to produce the product, increase yields and reduce the production of wastes.
History

Greek language
Greek is an independent branch of the Indo-European family of languages. Native to the southern Balkans, it has the longest documented history of any Indo-European language, spanning 34 centuries of written records. Its writing system has been the Greek alphabet for the majority of its history;...
Μεταβολισμός – "Metabolismos" for "change", or "overthrow". The history of the scientific study of metabolism spans several centuries and has moved from examining whole animals in early studies, to examining individual metabolic reactions in modern biochemistry. The first controlled experiment
Experiment
An experiment is a methodical procedure carried out with the goal of verifying, falsifying, or establishing the validity of a hypothesis. Experiments vary greatly in their goal and scale, but always rely on repeatable procedure and logical analysis of the results...
s in human metabolism were published by Santorio Santorio in 1614 in his book Ars de statica medicina. He described how he weighed himself before and after eating, sleep, working, sex, fasting, drinking, and excreting. He found that most of the food he took in was lost through what he called "insensible perspiration".
In these early studies, the mechanisms of these metabolic processes had not been identified and a vital force
Vitalism
Vitalism, as defined by the Merriam-Webster dictionary, is#a doctrine that the functions of a living organism are due to a vital principle distinct from biochemical reactions...
was thought to animate living tissue. In the 19th century, when studying the fermentation
Fermentation (food)
Fermentation in food processing typically is the conversion of carbohydrates to alcohols and carbon dioxide or organic acids using yeasts, bacteria, or a combination thereof, under anaerobic conditions. Fermentation in simple terms is the chemical conversion of sugars into ethanol...
of sugar to alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
by yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
, Louis Pasteur
Louis Pasteur
Louis Pasteur was a French chemist and microbiologist born in Dole. He is remembered for his remarkable breakthroughs in the causes and preventions of diseases. His discoveries reduced mortality from puerperal fever, and he created the first vaccine for rabies and anthrax. His experiments...
concluded that fermentation was catalyzed by substances within the yeast cells he called "ferments". He wrote that "alcoholic fermentation is an act correlated with the life and organization of the yeast cells, not with the death or putrefaction of the cells." This discovery, along with the publication by Friedrich Wöhler in 1828 of the chemical synthesis of urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
, notable for being the first organic compound prepared from wholly inorganic precursors, proved that the organic compounds and chemical reactions found in cells were no different in principle than any other part of chemistry.
It was the discovery of enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s at the beginning of the 20th century by Eduard Buchner
Eduard Buchner
Eduard Buchner was a German chemist and zymologist, awarded with the 1907 Nobel Prize in Chemistry thanks to his work on fermentation.-Early years:...
that separated the study of the chemical reactions of metabolism from the biological study of cells, and marked the beginnings of biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
. The mass of biochemical knowledge grew rapidly throughout the early 20th century. One of the most prolific of these modern biochemists was Hans Krebs
Hans Adolf Krebs
Sir Hans Adolf Krebs was a German-born British physician and biochemist. Krebs is best known for his identification of two important metabolic cycles: the urea cycle and the citric acid cycle...
who made huge contributions to the study of metabolism. He discovered the urea cycle and later, working with Hans Kornberg
Hans Kornberg
Professor Sir Hans Leo Kornberg, FRS is a British biochemist.-Early Life, Education and Career:Kornberg was born in 1928 in Germany of Jewish parents. In 1939 he left Nazi Germany , and moved to the care of an uncle in Yorkshire...
, the citric acid cycle and the glyoxylate cycle. Modern biochemical research has been greatly aided by the development of new techniques such as chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
, X-ray diffraction, NMR spectroscopy
NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei to determine physical and chemical properties of atoms or the molecules in which they are contained...
, radioisotopic labelling, electron microscopy
Electron microscope
An electron microscope is a type of microscope that uses a beam of electrons to illuminate the specimen and produce a magnified image. Electron microscopes have a greater resolving power than a light-powered optical microscope, because electrons have wavelengths about 100,000 times shorter than...
and molecular dynamics
Molecular dynamics
Molecular dynamics is a computer simulation of physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a period of time, giving a view of the motion of the atoms...
simulations. These techniques have allowed the discovery and detailed analysis of the many molecules and metabolic pathways in cells.
See also
- Anthropogenic metabolismAnthropogenic metabolism"Anthropogenic metabolism" is a term used in material flow analysis, substance flow analysis and waste management. It includes: "not only the physiological metabolism but also includes the thousands of goods and substances necessary to sustain modern life. Anthropogenic stands for man-made...
- Basal metabolic rateBasal metabolic rateBasal Metabolic Rate , and the closely related resting metabolic rate , is the amount of daily energy expended by humans and other animals at rest. Rest is defined as existing in a neutrally temperate environment while in the post-absorptive state...
- CalorimetryCalorimetryCalorimetry is the science of measuring the heat of chemical reactions or physical changes. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat...
- Inborn error of metabolismInborn error of metabolismInborn errors of metabolism comprise a large class of genetic diseases involving disorders of metabolism. The majority are due to defects of single genes that code for enzymes that facilitate conversion of various substances into others...
- Iron-sulfur world theoryIron-sulfur world theoryThe iron-sulfur world theory is a set of proposals for the origin of life and the early evolution of life advanced by Günter Wächtershäuser, a Munich patent lawyer with a degree in chemistry who had been encouraged and supported by philosopher Karl R. Popper to publish his ideas. The theory...
, a "metabolism first" theory of the origin of life. - RespirometryRespirometryRespirometry is a general term that encompass a number of techniques for obtaining estimates of the rates of metabolism of vertebrates, invertebrates, plants, tissues, cells, or microorganisms via an indirect measure of heat production ....
- Thermic effect of foodThermic effect of foodThermic effect of food , or TEF in shorthand, is the increment in energy expenditure above resting metabolic rate due to the cost of processing food for storage and use. It is one of the components of metabolism along with the resting metabolic rate, and the exercise component...
- Water metabolism
- Sulphur metabolism
- AntimetaboliteAntimetaboliteAn antimetabolite is a chemical that inhibits the use of a metabolite, which is another chemical that is part of normal metabolism. Such substances are often similar in structure to the metabolite that they interfere with, such as the antifolates that interfere with the use of folic acid...
Further reading
Introductory and , The Chemistry of Life. (Penguin Press Science, 1999), ISBN 0-14-027273-9 and , Into the Cool: Energy Flow, Thermodynamics, and Life. (University Of Chicago Press, 2005), ISBN 0-226-73936-8, Oxygen: The Molecule that Made the World. (Oxford University Press, USA, 2004), ISBN 0-19-860783-0Advanced and , Fundamentals of Enzymology: Cell and Molecular Biology of Catalytic Proteins. (Oxford University Press, 1999), ISBN 0-19-850229-X and , Biochemistry. (W. H. Freeman and Company, 2002), ISBN 0-7167-4955-6 and , Lehninger Principles of Biochemistry. (Palgrave Macmillan, 2004), ISBN 0-7167-4339-6 and , Brock's Biology of Microorganisms. (Benjamin Cummings, 2002), ISBN 0-13-066271-2 and , The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. (Clarendon Press, 1991), ISBN 0-19-855598-9 and , Bioenergetics. (Academic Press Inc., 2002), ISBN 0-12-518121-3

