
Fatty acid synthase
Encyclopedia
Fatty acid synthase is an enzyme
that in humans is encoded by the FASN gene
.
Fatty acid synthase is a multi-enzyme protein that catalyzes fatty acid synthesis
. It is not a single enzyme
but a whole enzymatic system composed of two identical 272 kDa multifunctional polypeptides, in which substrate
s are handed from one functional domain to the next.
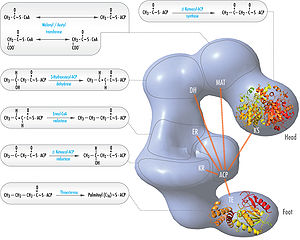
Its main function is to catalyze the synthesis of palmitate
from acetyl-CoA
and malonyl-CoA
, in the presence of NADPH
, into long-chain saturated fatty acid
s.
s and other biologically important molecules. They are synthesized by a series of decarboxylative Claisen condensation
reactions from acetyl-CoA
and malonyl-CoA
. Following each round of elongation the beta keto group is reduced to the fully saturated carbon chain by the sequential action of a ketoreductase (KR), dehydratase
(DH), and enol reductase
(ER). The growing fatty acid chain is carried between these active sites while attached covalently to the phosphopantetheine prosthetic group of an acyl carrier protein
(ACP), and is released by the action of a thioesterase
(TE) upon reaching a carbon chain length of 16 (palmitidic acid).
The mechanism of FAS I and FAS II elongation and reduction is the same, as the domains of the FAS II enzymes are largely homologous to their domain counterparts in FAS I multienzyme polypeptides. However, the differences in the organization of the enzymes - integrated in FAS I, discrete in FAS II - gives rise to many important biochemical differences.
The evolutionary history of fatty acid synthases are very much intertwined with that of polyketide synthase
s (PKS). Polyketide synthases use a similar mechanism and homologous domains to produce secondary metabolite lipids. Furthermore, polyketide synthases also exhibit a Type I and Type II organization. FAS I in animals is thought to have arisen through modification of PKS I in fungi, whereas FAS I in fungi and the CMN group of bacteria seem to have arisen separately through the fusion of FAS II genes.
The conventional model for organization of FAS (see the 'head-to-tail' model on the right) is largely based on the observations that the bifunctional reagent 1,3-dibromopropanone (DBP) is able to crosslink the active site cysteine
thiol of the KS domain in one FAS monomer with the phosphopantetheine
prosthetic group of the ACP domain in the other monomer. Complementation analysis of FAS dimers carrying different mutations on each monomer has established that the KS and MAT domains can cooperate with the ACP of either monomer. and a reinvestigation of the DBP crosslinking experiments revealed that the KS active site Cys161 thiol could be crosslinked to the ACP 4'-phosphopantetheine
thiol of either monomer. In addition, it has been recently reported that a heterodimeric FAS containing only one competent monomer is capable of palmitate synthesis.
The above observations seemed incompatible with the classical 'head-to-tail' model for FAS organization, and an alternative model has been proposed, predicting that the KS and MAT domains of both monomers lie closer to the center of the FAS dimer, where they can access the ACP of either subunit (see figure on the top right).
Recently, the elucidation of the low resolution X-ray crystallography structure of both pig (homodimer) and yeast FAS (heterododecamer) has provided key structural and mechanistic insights into this important enzyme.
and homeostasis
of fatty acid synthase is transcriptionally regulated by Upstream Stimulatory Factors (USF1
and USF2
) and sterol regulatory element binding protein
-1c (SREBP-1c) in response to feeding/insulin in living animals.
Although liver X receptor
(LXRs) modulate the expression of sterol regulatory element binding protein
-1c (SREBP-1c) in feeding, regulation of FAS by SREBP-1c is USF-dependent.
. FAS is up-regulated in breast cancers and as well as being an indicator of poor prognosis may also be worthwhile as a chemotherapeutic target. FAS may also be involved in the production of an endogenous ligand for the nuclear receptor PPARalpha
, the target of the fibrate
drugs for hyperlipidemia, and is being investigated as a possible drug target for treating the metabolic syndrome.
In some cancer cell lines, this protein has been found to be fused with estrogen receptor alpha
(ER-alpha), in which the N-terminus of FAS is fused in-frame with the C-terminus of ER-alpha.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that in humans is encoded by the FASN gene
Gene
A gene is a molecular unit of heredity of a living organism. It is a name given to some stretches of DNA and RNA that code for a type of protein or for an RNA chain that has a function in the organism. Living beings depend on genes, as they specify all proteins and functional RNA chains...
.
Fatty acid synthase is a multi-enzyme protein that catalyzes fatty acid synthesis
Fatty acid synthesis
Fatty acid synthesis is the creation of fatty acids from acetyl-CoA and malonyl-CoA precursors through action of enzymes called fatty acid synthases...
. It is not a single enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
but a whole enzymatic system composed of two identical 272 kDa multifunctional polypeptides, in which substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
s are handed from one functional domain to the next.
Its main function is to catalyze the synthesis of palmitate
Palmitic acid
Palmitic acid, or hexadecanoic acid in IUPAC nomenclature, is one of the most common saturated fatty acids found in animals and plants. Its molecular formula is CH314CO2H. As its name indicates, it is a major component of the oil from palm trees . Palmitate is a term for the salts and esters of...
from acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
and malonyl-CoA
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative.-Functions:It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis....
, in the presence of NADPH
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
, into long-chain saturated fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s.
Metabolic function
Fatty acids are aliphatic acids fundamental to energy production and storage, cellular structure and as intermediates in the biosynthesis of hormoneHormone
A hormone is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. In essence, it is a chemical messenger that transports a signal from one...
s and other biologically important molecules. They are synthesized by a series of decarboxylative Claisen condensation
Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone...
reactions from acetyl-CoA
Acetyl-CoA
Acetyl coenzyme A or acetyl-CoA is an important molecule in metabolism, used in many biochemical reactions. Its main function is to convey the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production. In chemical structure, acetyl-CoA is the thioester...
and malonyl-CoA
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative.-Functions:It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis....
. Following each round of elongation the beta keto group is reduced to the fully saturated carbon chain by the sequential action of a ketoreductase (KR), dehydratase
Dehydratase
Dehydratase is an enzyme that catalyzes the removal of oxygen and hydrogen from organic compounds in the form of water. This process is also known as dehydration.There are four classes of dehydratases:...
(DH), and enol reductase
Enoyl-acyl carrier protein reductase
Enoyl-acyl carrier protein reductase , is a key enzyme of the type II fatty acid synthesis system. ENR is an attractive target for narrow-spectrum antibacterial drug discovery because of its essential role in metabolism and its sequence conservation across many bacterial species...
(ER). The growing fatty acid chain is carried between these active sites while attached covalently to the phosphopantetheine prosthetic group of an acyl carrier protein
Acyl carrier protein
The acyl carrier protein is an important component in both fatty acid and polyketide biosynthesis with the growing chain bound during synthesis as a thiol ester at the distal thiol of a 4'-phosphopantethiene moiety...
(ACP), and is released by the action of a thioesterase
Thioesterase
Thioesterases are enzymes which belong to the Esterase family. Esterases, in turn, are one type of the several hydrolases known.Thioesterases exhibit Esterase activity specifically at a thiol group.Thioesterases or thiolester hydrolases are identified as members of E.C.3.1.2.-Examples:Acetyl-coA...
(TE) upon reaching a carbon chain length of 16 (palmitidic acid).
Classes
There are two principal classes of fatty acid synthases.- Type I systems utilise a single large, multifunctional polypeptide and are common to both mammals and fungi (although the structural arrangement of fungal and mammalian synthases differ). A Type I fatty acid synthase system is also found in the CMN group of bacteria (corynebacteria, mycobacteria, and nocardia). In these bacteria, the FAS I system produces palmititic acid, and cooperates with the FAS II system to produce a greater diversity of lipid products.
- Type II is found in archaeabacterial and eubacterial, and is characterized by the use of discrete, monofunctional enzymes for fatty acid synthesis. Inhibitors of this pathway (FASII) are being investigated as possible antibiotics.
The mechanism of FAS I and FAS II elongation and reduction is the same, as the domains of the FAS II enzymes are largely homologous to their domain counterparts in FAS I multienzyme polypeptides. However, the differences in the organization of the enzymes - integrated in FAS I, discrete in FAS II - gives rise to many important biochemical differences.
The evolutionary history of fatty acid synthases are very much intertwined with that of polyketide synthase
Polyketide synthase
Polyketide synthases are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages...
s (PKS). Polyketide synthases use a similar mechanism and homologous domains to produce secondary metabolite lipids. Furthermore, polyketide synthases also exhibit a Type I and Type II organization. FAS I in animals is thought to have arisen through modification of PKS I in fungi, whereas FAS I in fungi and the CMN group of bacteria seem to have arisen separately through the fusion of FAS II genes.
Structure
Mammalian FAS consists of a homodimer of two identical protein subunits, in which three catalytic domains in the N-terminal section (-ketoacyl synthase (KS), malonyl/acetyltransferase (MAT), and dehydrase (DH)), are separated by a core region of 600 residues from four C-terminal domains (enoyl reductase (ER), -ketoacyl reductase (KR), acyl carrier protein (ACP) and thioesterase (TE)).The conventional model for organization of FAS (see the 'head-to-tail' model on the right) is largely based on the observations that the bifunctional reagent 1,3-dibromopropanone (DBP) is able to crosslink the active site cysteine
Cysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
thiol of the KS domain in one FAS monomer with the phosphopantetheine
Phosphopantetheine
4'-Phosphopantetheine is an essential prosthetic group of acyl carrier protein and peptidyl carrier proteins and aryl carrier proteins derived from Coenzyme A...
prosthetic group of the ACP domain in the other monomer. Complementation analysis of FAS dimers carrying different mutations on each monomer has established that the KS and MAT domains can cooperate with the ACP of either monomer. and a reinvestigation of the DBP crosslinking experiments revealed that the KS active site Cys161 thiol could be crosslinked to the ACP 4'-phosphopantetheine
Phosphopantetheine
4'-Phosphopantetheine is an essential prosthetic group of acyl carrier protein and peptidyl carrier proteins and aryl carrier proteins derived from Coenzyme A...
thiol of either monomer. In addition, it has been recently reported that a heterodimeric FAS containing only one competent monomer is capable of palmitate synthesis.
The above observations seemed incompatible with the classical 'head-to-tail' model for FAS organization, and an alternative model has been proposed, predicting that the KS and MAT domains of both monomers lie closer to the center of the FAS dimer, where they can access the ACP of either subunit (see figure on the top right).
Recently, the elucidation of the low resolution X-ray crystallography structure of both pig (homodimer) and yeast FAS (heterododecamer) has provided key structural and mechanistic insights into this important enzyme.
 |
 |
Regulation
MetabolismMetabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
and homeostasis
Homeostasis
Homeostasis is the property of a system that regulates its internal environment and tends to maintain a stable, constant condition of properties like temperature or pH...
of fatty acid synthase is transcriptionally regulated by Upstream Stimulatory Factors (USF1
USF1
Upstream stimulatory factor 1 is a protein that in humans is encoded by the USF1 gene.-Interactions:USF1 has been shown to interact with USF2, FOSL1 and GTF2I.-External links:...
and USF2
USF2
Upstream stimulatory factor 2 is a protein that in humans is encoded by the USF2 gene.-Interactions:USF2 has been shown to interact with USF1 , PPRC1 and BRCA1.- Regulation :The USF2 gene is repressed by the microRNA miR-10a....
) and sterol regulatory element binding protein
Sterol regulatory element binding protein
Sterol Regulatory Element-Binding Proteins are transcription factors that bind to the sterol regulatory element DNA sequence TCACNCCAC. Mammalian SREBPs are encoded by the genes SREBF1 and SREBF2. SREBPs belong to the basic-helix-loop-helix leucine zipper class of transcription factors...
-1c (SREBP-1c) in response to feeding/insulin in living animals.
Although liver X receptor
Liver X receptor
The liver X receptor is a member of the nuclear receptor family of transcription factors and is closely related to nuclear receptors such as the PPARs, FXR and RXR. Liver X receptors are important regulators of cholesterol, fatty acid, and glucose homeostasis...
(LXRs) modulate the expression of sterol regulatory element binding protein
Sterol regulatory element binding protein
Sterol Regulatory Element-Binding Proteins are transcription factors that bind to the sterol regulatory element DNA sequence TCACNCCAC. Mammalian SREBPs are encoded by the genes SREBF1 and SREBF2. SREBPs belong to the basic-helix-loop-helix leucine zipper class of transcription factors...
-1c (SREBP-1c) in feeding, regulation of FAS by SREBP-1c is USF-dependent.
Clinical significance
FAS has been investigated as a possible oncogeneOncogene
An oncogene is a gene that has the potential to cause cancer. In tumor cells, they are often mutated or expressed at high levels.An oncogene is a gene found in the chromosomes of tumor cells whose activation is associated with the initial and continuing conversion of normal cells into cancer...
. FAS is up-regulated in breast cancers and as well as being an indicator of poor prognosis may also be worthwhile as a chemotherapeutic target. FAS may also be involved in the production of an endogenous ligand for the nuclear receptor PPARalpha
Peroxisome proliferator-activated receptor alpha
Peroxisome proliferator-activated receptor alpha , also known as NR1C1 , is a nuclear receptor protein that in humans is encoded by the PPARA gene.- Function :...
, the target of the fibrate
Fibrate
In pharmacology, the fibrates are a class of amphipathic carboxylic acids. They are used for a range of metabolic disorders, mainly hypercholesterolemia , and are therefore hypolipidemic agents.- Members :...
drugs for hyperlipidemia, and is being investigated as a possible drug target for treating the metabolic syndrome.
In some cancer cell lines, this protein has been found to be fused with estrogen receptor alpha
Estrogen receptor alpha
Estrogen receptor alpha , also known as NR3A1 , is a nuclear receptor that is activated by the sex hormone estrogen...
(ER-alpha), in which the N-terminus of FAS is fused in-frame with the C-terminus of ER-alpha.
See also
- Fatty acid synthesisFatty acid synthesisFatty acid synthesis is the creation of fatty acids from acetyl-CoA and malonyl-CoA precursors through action of enzymes called fatty acid synthases...
- Fatty acid metabolismFatty acid metabolismFatty acids are an important source of energy and adenosine triphosphate for many cellular organisms. Excess fatty acids, glucose, and other nutrients can be stored efficiently as fat. Triglycerides yield more than twice as much energy for the same mass as do carbohydrates or proteins. All cell...
- Fatty acid degradationFatty acid degradationFatty acid degradation is the process in which fatty acids are broken down into their metabolites, in the end generating acetyl-CoA, the entry molecule for the citric acid cycle, the main energy supply of animals...
- Fatty acidFatty acidIn chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
- Essential fatty acidEssential fatty acidEssential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health but cannot synthesize them...
- Enoyl-acyl carrier protein reductaseEnoyl-acyl carrier protein reductaseEnoyl-acyl carrier protein reductase , is a key enzyme of the type II fatty acid synthesis system. ENR is an attractive target for narrow-spectrum antibacterial drug discovery because of its essential role in metabolism and its sequence conservation across many bacterial species...
- List of fatty acid metabolism disorders
External links
- http://web.indstate.edu/thcme/mwking/lipid-synthesis.html#synthesis
- http://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb2/part1/fasynthesis.htm

