Fat
Encyclopedia
Fats consist of a wide group of compounds that are generally soluble in organic solvents and generally insoluble in water. Chemically
, fats are triglyceride
s, triesters
of glycerol
and any of several fatty acid
s. Fats may be either solid
or liquid
at room temperature
, depending on their structure and composition. Although the words "oils", "fats", and "lipid
s" are all used to refer to fats, "oils" is usually used to refer to fats that are liquids at normal room temperature, while "fats" is usually used to refer to fats that are solids at normal room temperature. "Lipids" is used to refer to both liquid and solid fats, along with other related substances, usually in a medical or biochemical context. The word "oil
" is also used for any substance that does not mix with water and has a greasy feel, such as petroleum
(or crude oil), heating oil
, and essential oils, regardless of its chemical structure.
Fats form a category of lipid
, distinguished from other lipids by their chemical structure
and physical properties. This category of molecules is important for many forms of life, serving both structural and metabolic functions. They are an important part of the diet
of most heterotroph
s (including humans). Fats or lipids are broken down in the body by enzymes called lipase
s produced in the pancreas
.
Examples of edible animal fats are lard
, fish oil
, butter
/ghee
and whale blubber. They are obtained from fats in the milk and meat, as well as from under the skin, of an animal. Examples of edible plant fats
include peanut, soya bean, sunflower, sesame, coconut and olive oils, and cocoa butter
. Vegetable shortening, used mainly for baking, and margarine
, used in baking and as a spread, can be derived from the above oils by hydrogenation
.
These examples of fats can be categorized into saturated fat
s and unsaturated fat
s. Unsaturated fats can be further divided into cis fats, which are the most common in nature, and trans fat
s, which are rare in nature but present in partially-hydrogenated vegetable oils.
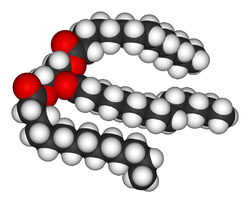 There are many different kinds of fats, but each is a variation on the same chemical structure. All fats are derivatives of fatty acid
There are many different kinds of fats, but each is a variation on the same chemical structure. All fats are derivatives of fatty acid
s and glycerol
. The molecules are called triglycerides, which are triester
s of glycerol (an ester being the molecule formed from the reaction of the carboxylic acid and an organic alcohol). As a simple visual illustration, if the kinks and angles of these chains were straightened out, the molecule would have the shape of a capital letter E. The fatty acids would each be a horizontal line; the glycerol "backbone" would be the vertical line that joins the horizontal lines. Fats therefore have "ester" bonds
.
The properties of any specific fat molecule depend on the particular fatty acids that constitute it. Different fatty acids are composed of different numbers of carbon and hydrogen atoms. The carbon atoms, each bonded to two neighboring carbon atoms, form a zigzagging chain; the more carbon atoms there are in any fatty acid, the longer its chain will be. Fatty acids with long chains are more susceptible to intermolecular forces of attraction (in this case, van der Waals forces), raising its melting point
. Long chains also yield more energy
per molecule when metabolized.
. Values of n usually range from 13 to 17. Each carbon atom in the chain is saturated with hydrogen, meaning they are bonded
to as many hydrogens as possible. In unsaturated fats are derived from fatty acids with the formula CnH(2n-1)CO2H. These fatty acids contain double bond
s within carbon chain. This results in an "unsaturated" fatty acid. More specifically, it would be a monounsaturated fatty acid. A polyunsaturated fatty acid
would be a fatty acid with more than one double bond; they have the formula , CnH(2n-3)CO2H. and CnH(2n-5)CO2H. Unsaturated fats can be converted to saturated ones by the process of hydrogenation
. This technology underpinned the development of margerine.
Saturated and unsaturated fats differ in their energy content and melting point. Since an unsaturated fat contains fewer carbon-hydrogen bonds than a saturated fat with the same number of carbon atoms, unsaturated fats will yield slightly less energy during metabolism than saturated fats with the same number of carbon atoms. Saturated fats can stack themselves in a closely packed arrangement, so they can freeze easily and are typically solid at room temperature. For example, animal fats tallow
and lard
are high in saturated fatty acid content and are solids. Olive and linseed oils on the other hand are highly unsaturated and are oily.
s) are commercially produced. Trans fatty acids are rare in nature. The cis-isomer introduces a kink into the molecule that prevents the fats from stacking efficiently as in the case of fats with saturated chains. This decreases intermolecular forces between the fat molecules, making it more difficult for unsaturated cis-fats to freeze; they are typically liquid at room temperature. Trans fats may still stack like saturated fats, and are not as susceptible to metabolization as other fats. Trans fats may significantly increase the risk of coronary heart disease
.
s A
, D
, E
, and K
are fat-soluble, meaning they can only be digested, absorbed, and transported in conjunction with fats. Fats are also sources of essential fatty acid
s, an important dietary requirement.
Fats play a vital role in maintaining healthy skin
and hair
, insulating body organs against shock, maintaining body temperature, and promoting healthy cell function.
Fats also serve as energy stores for the body, containing about 37.8 kilojoule
s (9 calorie
s) per gram
of fat. They are broken down in the body to release glycerol and free fatty acid
s. The glycerol can be converted to glucose
by the liver and thus used as a source of energy.
Fat also serves as a useful buffer towards a host of diseases. When a particular substance, whether chemical or biotic—reaches unsafe levels in the bloodstream, the body can effectively dilute—or at least maintain equilibrium of—the offending substances by storing it in new fat tissue. This helps to protect vital organs, until such time as the offending substances can be metabolized and/or removed from the body by such means as excretion
, urination
, accidental or intentional bloodletting
, sebum excretion, and hair
growth.
While it is nearly impossible to remove fat completely from the diet, it would also be unhealthy to do so. Some fatty acids are essential nutrients, meaning that they can't be produced in the body from other compounds and need to be consumed in small amounts. All other fats required by the body are non-essential and can be produced in the body from other compounds.
In animals, adipose
, or fatty tissue is the body's means of storing metabolic energy over extended periods of time. Depending on current physiological conditions, adipocytes store fat derived from the diet and liver metabolism
or degrade stored fat to supply fatty acids and glycerol
to the circulation
. These metabolic activities are regulated by several hormones (i.e., insulin
, glucagon
and epinephrine
). The location of the tissue determines its metabolic profile: "visceral fat" is located within the abdominal wall (i.e., beneath the wall of abdominal muscle) whereas "subcutaneous fat" is located beneath the skin (and includes fat that is located in the abdominal area beneath the skin but above the abdominal muscle wall). Visceral fat was recently discovered to be a significant producer of signaling chemicals (i.e., hormone
s), among which are several which are involved in inflammatory tissue responses. One of these is resistin
which has been linked to obesity, insulin resistance, and Type 2 diabetes. This latter result is currently controversial, and there have been reputable studies supporting all sides on the issue.
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
, fats are triglyceride
Triglyceride
A triglyceride is an ester derived from glycerol and three fatty acids. There are many triglycerides, depending on the oil source, some are highly unsaturated, some less so....
s, triesters
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
of glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
and any of several fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s. Fats may be either solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
or liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
, depending on their structure and composition. Although the words "oils", "fats", and "lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
s" are all used to refer to fats, "oils" is usually used to refer to fats that are liquids at normal room temperature, while "fats" is usually used to refer to fats that are solids at normal room temperature. "Lipids" is used to refer to both liquid and solid fats, along with other related substances, usually in a medical or biochemical context. The word "oil
Oil (disambiguation)
In the most general sense, an oil is any liquid not miscible with water.Oil usually refers to:* Petroleum , a fossil fuel* Lubricants or lubrication* Edible oil or cooking oil* Motor oil* Fuel oil or Heating oil or Lighting oil...
" is also used for any substance that does not mix with water and has a greasy feel, such as petroleum
Petroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
(or crude oil), heating oil
Heating oil
Heating oil, or oil heat, is a low viscosity, flammable liquid petroleum product used as a fuel for furnaces or boilers in buildings. Home heating oil is often abbreviated as HHO...
, and essential oils, regardless of its chemical structure.
Fats form a category of lipid
Lipid
Lipids constitute a broad group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins , monoglycerides, diglycerides, triglycerides, phospholipids, and others...
, distinguished from other lipids by their chemical structure
Chemical structure
A chemical structure includes molecular geometry, electronic structure and crystal structure of molecules. Molecular geometry refers to the spatial arrangement of atoms in a molecule and the chemical bonds that hold the atoms together. Molecular geometry can range from the very simple, such as...
and physical properties. This category of molecules is important for many forms of life, serving both structural and metabolic functions. They are an important part of the diet
Diet (nutrition)
In nutrition, diet is the sum of food consumed by a person or other organism. Dietary habits are the habitual decisions an individual or culture makes when choosing what foods to eat. With the word diet, it is often implied the use of specific intake of nutrition for health or weight-management...
of most heterotroph
Heterotroph
A heterotroph is an organism that cannot fix carbon and uses organic carbon for growth. This contrasts with autotrophs, such as plants and algae, which can use energy from sunlight or inorganic compounds to produce organic compounds such as carbohydrates, fats, and proteins from inorganic carbon...
s (including humans). Fats or lipids are broken down in the body by enzymes called lipase
Lipase
A lipase is an enzyme that catalyzes the formation or cleavage of fats . Lipases are a subclass of the esterases.Lipases perform essential roles in the digestion, transport and processing of dietary lipids in most, if not all, living organisms...
s produced in the pancreas
Pancreas
The pancreas is a gland organ in the digestive and endocrine system of vertebrates. It is both an endocrine gland producing several important hormones, including insulin, glucagon, and somatostatin, as well as a digestive organ, secreting pancreatic juice containing digestive enzymes that assist...
.
Examples of edible animal fats are lard
Lard
Lard is pig fat in both its rendered and unrendered forms. Lard was commonly used in many cuisines as a cooking fat or shortening, or as a spread similar to butter. Its use in contemporary cuisine has diminished because of health concerns posed by its saturated-fat content and its often negative...
, fish oil
Fish oil
Fish oil is oil derived from the tissues of oily fish. Fish oils contain the omega-3 fatty acids eicosapentaenoic acid , and docosahexaenoic acid , precursors of certain eicosanoids that are known to reduce inflammation throughout the body, and are thought to have many health benefits.Fish do not...
, butter
Butter
Butter is a dairy product made by churning fresh or fermented cream or milk. It is generally used as a spread and a condiment, as well as in cooking applications, such as baking, sauce making, and pan frying...
/ghee
Ghee
Ghee is a class of clarified butter that originated in South Asia and is commonly used in South Asian cuisine....
and whale blubber. They are obtained from fats in the milk and meat, as well as from under the skin, of an animal. Examples of edible plant fats
Vegetable fats and oils
Vegetable fats and oils are lipid materials derived from plants. Physically, oils are liquid at room temperature, and fats are solid. Chemically, both fats and oils are composed of triglycerides, as contrasted with waxes which lack glycerin in their structure...
include peanut, soya bean, sunflower, sesame, coconut and olive oils, and cocoa butter
Cocoa butter
Cocoa butter, also called theobroma oil, is a pale-yellow, pure edible vegetable fat extracted from the cocoa bean. It is used to make chocolate, biscuits, and baked goods, as well as some pharmaceuticals, ointments, and toiletries...
. Vegetable shortening, used mainly for baking, and margarine
Margarine
Margarine , as a generic term, can indicate any of a wide range of butter substitutes, typically composed of vegetable oils. In many parts of the world, the market share of margarine and spreads has overtaken that of butter...
, used in baking and as a spread, can be derived from the above oils by hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
.
These examples of fats can be categorized into saturated fat
Saturated fat
Saturated fat is fat that consists of triglycerides containing only saturated fatty acids. Saturated fatty acids have no double bonds between the individual carbon atoms of the fatty acid chain. That is, the chain of carbon atoms is fully "saturated" with hydrogen atoms...
s and unsaturated fat
Unsaturated fat
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the fatty acid chain. A fat molecule is monounsaturated if it contains one double bond, and polyunsaturated if it contains more than one double bond. Where double bonds are formed, hydrogen atoms are...
s. Unsaturated fats can be further divided into cis fats, which are the most common in nature, and trans fat
Trans fat
Trans fat is the common name for unsaturated fat with trans-isomer fatty acid. Because the term refers to the configuration of a double carbon-carbon bond, trans fats are sometimes monounsaturated or polyunsaturated, but never saturated....
s, which are rare in nature but present in partially-hydrogenated vegetable oils.
Chemical structure

Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s and glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
. The molecules are called triglycerides, which are triester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s of glycerol (an ester being the molecule formed from the reaction of the carboxylic acid and an organic alcohol). As a simple visual illustration, if the kinks and angles of these chains were straightened out, the molecule would have the shape of a capital letter E. The fatty acids would each be a horizontal line; the glycerol "backbone" would be the vertical line that joins the horizontal lines. Fats therefore have "ester" bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
.
The properties of any specific fat molecule depend on the particular fatty acids that constitute it. Different fatty acids are composed of different numbers of carbon and hydrogen atoms. The carbon atoms, each bonded to two neighboring carbon atoms, form a zigzagging chain; the more carbon atoms there are in any fatty acid, the longer its chain will be. Fatty acids with long chains are more susceptible to intermolecular forces of attraction (in this case, van der Waals forces), raising its melting point
Melting point
The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure...
. Long chains also yield more energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
per molecule when metabolized.
Saturated and unsaturated fats
A fat's constituent fatty acids may also differ in the C/H ratio. When all three fatty acids have the formula CnH(2n+1)CO2H, the resulting fat is called "saturated"Saturated fat
Saturated fat is fat that consists of triglycerides containing only saturated fatty acids. Saturated fatty acids have no double bonds between the individual carbon atoms of the fatty acid chain. That is, the chain of carbon atoms is fully "saturated" with hydrogen atoms...
. Values of n usually range from 13 to 17. Each carbon atom in the chain is saturated with hydrogen, meaning they are bonded
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
to as many hydrogens as possible. In unsaturated fats are derived from fatty acids with the formula CnH(2n-1)CO2H. These fatty acids contain double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
s within carbon chain. This results in an "unsaturated" fatty acid. More specifically, it would be a monounsaturated fatty acid. A polyunsaturated fatty acid
Polyunsaturated fatty acid
Polyunsaturated fatty acids are fatty acids that contain more than one double bond in their backbone. This class includes many important compounds, such as essential fatty acids and those that give drying oils their characteristic property....
would be a fatty acid with more than one double bond; they have the formula , CnH(2n-3)CO2H. and CnH(2n-5)CO2H. Unsaturated fats can be converted to saturated ones by the process of hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
. This technology underpinned the development of margerine.
Saturated and unsaturated fats differ in their energy content and melting point. Since an unsaturated fat contains fewer carbon-hydrogen bonds than a saturated fat with the same number of carbon atoms, unsaturated fats will yield slightly less energy during metabolism than saturated fats with the same number of carbon atoms. Saturated fats can stack themselves in a closely packed arrangement, so they can freeze easily and are typically solid at room temperature. For example, animal fats tallow
Tallow
Tallow is a rendered form of beef or mutton fat, processed from suet. It is solid at room temperature. Unlike suet, tallow can be stored for extended periods without the need for refrigeration to prevent decomposition, provided it is kept in an airtight container to prevent oxidation.In industry,...
and lard
Lard
Lard is pig fat in both its rendered and unrendered forms. Lard was commonly used in many cuisines as a cooking fat or shortening, or as a spread similar to butter. Its use in contemporary cuisine has diminished because of health concerns posed by its saturated-fat content and its often negative...
are high in saturated fatty acid content and are solids. Olive and linseed oils on the other hand are highly unsaturated and are oily.
Trans fats
There are two ways the double bond may be arranged: the isomer with both parts of the chain on the same side of the double bond (the cis-isomer), or the isomer with the parts of the chain on opposite sides of the double bond (the trans-isomer). Most trans-isomer fats (commonly called trans fatTrans fat
Trans fat is the common name for unsaturated fat with trans-isomer fatty acid. Because the term refers to the configuration of a double carbon-carbon bond, trans fats are sometimes monounsaturated or polyunsaturated, but never saturated....
s) are commercially produced. Trans fatty acids are rare in nature. The cis-isomer introduces a kink into the molecule that prevents the fats from stacking efficiently as in the case of fats with saturated chains. This decreases intermolecular forces between the fat molecules, making it more difficult for unsaturated cis-fats to freeze; they are typically liquid at room temperature. Trans fats may still stack like saturated fats, and are not as susceptible to metabolization as other fats. Trans fats may significantly increase the risk of coronary heart disease
Coronary heart disease
Coronary artery disease is the end result of the accumulation of atheromatous plaques within the walls of the coronary arteries that supply the myocardium with oxygen and nutrients. It is sometimes also called coronary heart disease...
.
Importance for living organisms
VitaminVitamin
A vitamin is an organic compound required as a nutrient in tiny amounts by an organism. In other words, an organic chemical compound is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on...
s A
Vitamin A
Vitamin A is a vitamin that is needed by the retina of the eye in the form of a specific metabolite, the light-absorbing molecule retinal, that is necessary for both low-light and color vision...
, D
Vitamin D
Vitamin D is a group of fat-soluble secosteroids. In humans, vitamin D is unique both because it functions as a prohormone and because the body can synthesize it when sun exposure is adequate ....
, E
Vitamin E
Vitamin E is used to refer to a group of fat-soluble compounds that include both tocopherols and tocotrienols. There are many different forms of vitamin E, of which γ-tocopherol is the most common in the North American diet. γ-Tocopherol can be found in corn oil, soybean oil, margarine and dressings...
, and K
Vitamin K
Vitamin K is a group of structurally similar, fat soluble vitamins that are needed for the posttranslational modification of certain proteins required for blood coagulation and in metabolic pathways in bone and other tissue. They are 2-methyl-1,4-naphthoquinone derivatives...
are fat-soluble, meaning they can only be digested, absorbed, and transported in conjunction with fats. Fats are also sources of essential fatty acid
Essential fatty acid
Essential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health but cannot synthesize them...
s, an important dietary requirement.
Fats play a vital role in maintaining healthy skin
Skin
-Dermis:The dermis is the layer of skin beneath the epidermis that consists of connective tissue and cushions the body from stress and strain. The dermis is tightly connected to the epidermis by a basement membrane. It also harbors many Mechanoreceptors that provide the sense of touch and heat...
and hair
Hair
Hair is a filamentous biomaterial, that grows from follicles found in the dermis. Found exclusively in mammals, hair is one of the defining characteristics of the mammalian class....
, insulating body organs against shock, maintaining body temperature, and promoting healthy cell function.
Fats also serve as energy stores for the body, containing about 37.8 kilojoule
Joule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
s (9 calorie
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
s) per gram
Gram
The gram is a metric system unit of mass....
of fat. They are broken down in the body to release glycerol and free fatty acid
Fatty acid
In chemistry, especially biochemistry, a fatty acid is a carboxylic acid with a long unbranched aliphatic tail , which is either saturated or unsaturated. Most naturally occurring fatty acids have a chain of an even number of carbon atoms, from 4 to 28. Fatty acids are usually derived from...
s. The glycerol can be converted to glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
by the liver and thus used as a source of energy.
Fat also serves as a useful buffer towards a host of diseases. When a particular substance, whether chemical or biotic—reaches unsafe levels in the bloodstream, the body can effectively dilute—or at least maintain equilibrium of—the offending substances by storing it in new fat tissue. This helps to protect vital organs, until such time as the offending substances can be metabolized and/or removed from the body by such means as excretion
Excretion
Excretion is the process by which waste products of metabolism and other non-useful materials are eliminated from an organism. This is primarily carried out by the lungs, kidneys and skin. This is in contrast with secretion, where the substance may have specific tasks after leaving the cell...
, urination
Urination
Urination, also known as micturition, voiding, peeing, weeing, pissing, and more rarely, emiction, is the ejection of urine from the urinary bladder through the urethra to the outside of the body. In healthy humans the process of urination is under voluntary control...
, accidental or intentional bloodletting
Bloodletting
Bloodletting is the withdrawal of often little quantities of blood from a patient to cure or prevent illness and disease. Bloodletting was based on an ancient system of medicine in which blood and other bodily fluid were considered to be "humors" the proper balance of which maintained health...
, sebum excretion, and hair
Hair
Hair is a filamentous biomaterial, that grows from follicles found in the dermis. Found exclusively in mammals, hair is one of the defining characteristics of the mammalian class....
growth.
While it is nearly impossible to remove fat completely from the diet, it would also be unhealthy to do so. Some fatty acids are essential nutrients, meaning that they can't be produced in the body from other compounds and need to be consumed in small amounts. All other fats required by the body are non-essential and can be produced in the body from other compounds.
Adipose tissue

In animals, adipose
Adipose tissue
In histology, adipose tissue or body fat or fat depot or just fat is loose connective tissue composed of adipocytes. It is technically composed of roughly only 80% fat; fat in its solitary state exists in the liver and muscles. Adipose tissue is derived from lipoblasts...
, or fatty tissue is the body's means of storing metabolic energy over extended periods of time. Depending on current physiological conditions, adipocytes store fat derived from the diet and liver metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
or degrade stored fat to supply fatty acids and glycerol
Glycerol
Glycerol is a simple polyol compound. It is a colorless, odorless, viscous liquid that is widely used in pharmaceutical formulations. Glycerol has three hydroxyl groups that are responsible for its solubility in water and its hygroscopic nature. The glycerol backbone is central to all lipids...
to the circulation
Circulatory system
The circulatory system is an organ system that passes nutrients , gases, hormones, blood cells, etc...
. These metabolic activities are regulated by several hormones (i.e., insulin
Insulin
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
, glucagon
Glucagon
Glucagon, a hormone secreted by the pancreas, raises blood glucose levels. Its effect is opposite that of insulin, which lowers blood glucose levels. The pancreas releases glucagon when blood sugar levels fall too low. Glucagon causes the liver to convert stored glycogen into glucose, which is...
and epinephrine
Epinephrine
Epinephrine is a hormone and a neurotransmitter. It increases heart rate, constricts blood vessels, dilates air passages and participates in the fight-or-flight response of the sympathetic nervous system. In chemical terms, adrenaline is one of a group of monoamines called the catecholamines...
). The location of the tissue determines its metabolic profile: "visceral fat" is located within the abdominal wall (i.e., beneath the wall of abdominal muscle) whereas "subcutaneous fat" is located beneath the skin (and includes fat that is located in the abdominal area beneath the skin but above the abdominal muscle wall). Visceral fat was recently discovered to be a significant producer of signaling chemicals (i.e., hormone
Hormone
A hormone is a chemical released by a cell or a gland in one part of the body that sends out messages that affect cells in other parts of the organism. Only a small amount of hormone is required to alter cell metabolism. In essence, it is a chemical messenger that transports a signal from one...
s), among which are several which are involved in inflammatory tissue responses. One of these is resistin
Resistin
Resistin also known as adipose tissue-specific secretory factor or C/EBP-epsilon-regulated myeloid-specific secreted cysteine-rich protein is a cysteine-rich protein that in humans is encoded by the RETN gene....
which has been linked to obesity, insulin resistance, and Type 2 diabetes. This latter result is currently controversial, and there have been reputable studies supporting all sides on the issue.
See also
|
Omega-6 fatty acid n−6 fatty acids are a family of unsaturated fatty acids that have in common a final carbon–carbon double bond in the n−6 position, that is, the sixth bond, counting from the methyl end.The biological effects of the n−6 fatty acids are largely mediated by their conversion to n-6 eicosanoids... Vegetable fats and oils Vegetable fats and oils are lipid materials derived from plants. Physically, oils are liquid at room temperature, and fats are solid. Chemically, both fats and oils are composed of triglycerides, as contrasted with waxes which lack glycerin in their structure... Adipocyte However, in some reports and textbooks, the number of fat cell increased in childhood and adolescence. The total number is constant in both obese and lean adult... Yellow grease Yellow grease is derived from used cooking oil from the fast-food industry. It is used to feed livestock, and to make soap, make-up, clothes, rubber, detergents, and bio-diesel fuel.... National Association to Advance Fat Acceptance The National Association to Advance Fat Acceptance is a non-profit civil-rights organization in the US dedicated to improving the quality of life for the obese... |
Further reading
- Hayes, K.C. (May 2005). Dietary fat and blood lipids. Retrieved March 10, 2011.

