
ATP synthase
Encyclopedia
ATP synthase is an important enzyme
that provides energy for the cell to use through the synthesis of adenosine triphosphate
(ATP). ATP
is the most commonly used "energy currency" of cells from most organisms. It is formed from adenosine diphosphate
(ADP) and inorganic phosphate
(Pi) which releases energy
.
The overall reaction sequence is: ATP synthase + ADP + Pi → ATP Synthase + ATP
This energy is often in the form of protium or H+, moving down an electrochemical gradient
, such as from the lumen into the stroma of chloroplast
s or from the inter-membrane space into the matrix in mitochondria
.
The nomenclature
of the enzyme suffers from a long history. The F1 fraction derives its name from the term "Fraction 1" and FO (written as a subscript letter "o", not "zero") derives its name from being the oligomycin
binding fraction. Oligomycin
, an antibiotic, is able to inhibit the FO unit of ATP synthase.
These functional regions consist of different protein subunits - refer to tables.
s created by exposing mitochondria to ultrasound. This ATPase activity was further associated with the creation of ATP by a long series of experiments in many laboratories.
, e
, f
, g
, F6 and 8 (or A6L).
developed the binding change, or flip-flop, mechanism, which postulated that ATP synthesis is coupled with a conformational change in the ATP synthase generated by rotation of the gamma subunit. The research group of John E. Walker
, then at the MRC Laboratory of Molecular Biology in Cambridge but now at the MRC Mitochondrial Biology Unit (also in Cambridge), crystallized the F1 catalytic-domain of ATP synthase. The structure, at the time the largest asymmetric protein structure known, indicated that Boyer's rotary-catalysis model was, in essence, correct. For elucidating this, Boyer and Walker shared half of the 1997 Nobel Prize in Chemistry
. Jens Christian Skou
received the other half of the Chemistry prize that year "for the first discovery of an ion-transporting enzyme, Na+, K+ -ATPase."
 The crystal structure of the F1 showed alternating alpha and beta subunit
The crystal structure of the F1 showed alternating alpha and beta subunit
s (3 of each), arranged like segments of an orange around an asymmetrical gamma subunit. According to the current model of ATP synthesis (known as the alternating catalytic model), the proton-motive force across the inner mitochondrial membrane, generated by the electron transport chain, drives the passage of proton
s through the membrane via the FO region of ATP synthase. A portion of the FO (the ring of c-subunits
) rotates as the protons pass through the membrane. The c-ring
is tightly attached to the asymmetric central stalk (consisting primarily of the gamma subunit), which rotates within the alpha3beta3 of F1 causing the 3 catalytic nucleotide binding sites to go through a series of conformational changes that leads to ATP synthesis. The major F1 subunits are prevented from rotating in sympathy with the central stalk rotor by a peripheral stalk that joins the alpha3beta3 to the non-rotating portion of FO. The structure of the intact ATP synthase is currently known at low-resolution from electron cryo-microscopy (cryo-EM) studies of the complex. The cryo-EM model of ATP synthase suggests that the peripheral stalk is a flexible structure that wraps around the complex as it joins F1 to FO. Under the right conditions, the enzyme reaction can also be carried out in reverse, with ATP hydrolysis driving proton pumping across the membrane.
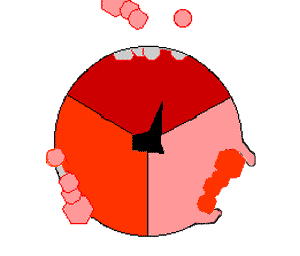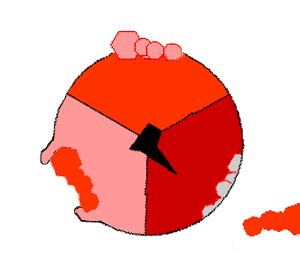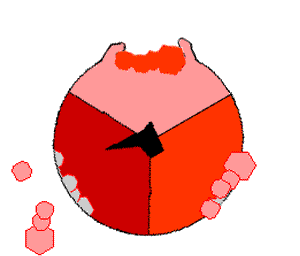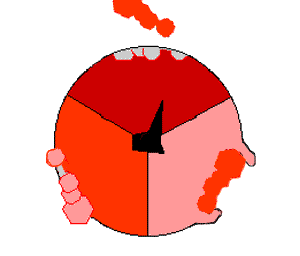
The binding change mechanism involves the active site of a β subunit cycling between three states. In the "open" state, ADP and phosphate enter the active site, in the diagram to the right this is shown in red. The protein then closes up around the molecules and binds them loosely - the "loose" state (shown in orange). The enzyme then undergoes another change in shape and forces these molecules together, with the active site in the resulting "tight" state (shown in pink) binding the newly-produced ATP molecule with very high affinity
. Finally, the active site cycles back to the open state, releasing ATP and binding more ADP and phosphate, ready for the next cycle of ATP production.
gradient
, this is used by fermenting bacteria that do not have an electron transport chain, and hydrolyze ATP to make a proton gradient, which they use for flagella and transport of nutrients into the cell.
In respiring bacteria
under physiological conditions, ATP synthase, in general, runs in the opposite direction, creating ATP while using the protonmotive force created by the electron transport chain as a source of energy. The overall process of creating energy in this fashion is termed oxidative phosphorylation
.
The same process takes place in the mitochondria, where ATP synthase is located in the inner mitochondrial membrane (so that F1-part sticks into mitochondrial matrix, where ATP synthesis takes place).
of ATP synthase is thought to be an example of modular evolution, where two subunits with their own functions have become associated and gained new functionality. This coupling must have occurred early in the evolution of life as evidenced by essentially the same structure and processes of ATP synthase enzymes conserved in all kingdoms of life. The F-ATP synthase shows large amounts of similarity both functionally and mechanically to the V-ATPase
. However whilst the F-ATP synthase generates ATP by utilising a proton gradient the V-ATPase
is responsible for generating a proton gradient at the expense of ATP, generating pH values as low as 1.
The F1 particle also shows significant similarity to hexameric DNA helicases and the FO particle shows some similarity to H+ powered flagellar motor
complexes. The α3β3 hexamer of the F1 particle shows significant structural similarity to hexameric DNA helicases; both form a ring with 3 fold rotational symmetry with a central pore. Both also have roles dependent on the relative rotation of a macromolecule within the pore; the DNA helicases use the helical shape of DNA to drive their motion along the DNA molecule and to detect supercoiling whilst the α3β3 hexamer uses the conformational changes due rotation of the γ subunit to drive an enzymatic reaction.
The H+ motor of the FO particle shows great functional similarity to the H+ motors seen in flagellar motors. Both feature a ring of many small alpha helical proteins that rotate relative to nearby stationary proteins using a H+ potential gradient as an energy source. This is, however, a fairly tenuous link - the overall structure of flagellar motors is far more complex than the FO particle and the ring of rotating proteins is far larger, with around 30 compared to the 10, 11, or 14 known in the FO complex.
The modular evolution theory for the origin of ATP synthase suggests that two subunits with independent function, a DNA helicase with ATPase activity and a H+ motor, were able to bind, and the rotation of the motor drive the ATPase activity of the helicase in reverse. This would then evolve to become more efficient, and eventually develop into the complex ATP synthases seen today. In alternative fashion, the DNA helicase/H+ motor complex may have had H+ pump activity, the ATPase activity of the helicase driving the H+ motor in reverse. This could later evolve to carry out the reverse reaction and act as an ATP synthase.
membrane; the CF1-part sticks into stroma
, where dark reactions of photosynthesis (Also called the light-independent reactions or the Calvin cycle
) and ATP synthesis take place. The overall structure and the catalytic mechanism of the chloroplast ATP synthase are almost the same as those of the mitochondrial enzyme. However, in chloroplasts, the proton motive force is generated not by respiratory electron transport chain but by primary photosynthetic proteins.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that provides energy for the cell to use through the synthesis of adenosine triphosphate
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP). ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
is the most commonly used "energy currency" of cells from most organisms. It is formed from adenosine diphosphate
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
(ADP) and inorganic phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
(Pi) which releases energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
.
The overall reaction sequence is: ATP synthase + ADP + Pi → ATP Synthase + ATP
This energy is often in the form of protium or H+, moving down an electrochemical gradient
Electrochemical gradient
An electrochemical gradient is a spatial variation of both electrical potential and chemical concentration across a membrane; that is, a combination of the membrane potential and the pH gradient...
, such as from the lumen into the stroma of chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s or from the inter-membrane space into the matrix in mitochondria
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
.
Structure
Located within the mitochondria ATP synthase consists of 2 regions- the FO portion is within the membrane.
- The F1 portion of the ATP synthase is above the membrane, inside the matrix of the mitochondria.

The nomenclature
Nomenclature
Nomenclature is a term that applies to either a list of names or terms, or to the system of principles, procedures and terms related to naming - which is the assigning of a word or phrase to a particular object or property...
of the enzyme suffers from a long history. The F1 fraction derives its name from the term "Fraction 1" and FO (written as a subscript letter "o", not "zero") derives its name from being the oligomycin
Oligomycin
Oligomycins are macrolides created by Streptomyces that can be poisonous to other organisms.-Function:They have use as antibiotics.In addition, oligomycin inhibits ATP synthase by blocking its proton channel , which is necessary for oxidative phosphorylation of ADP to ATP . The inhibition of ATP...
binding fraction. Oligomycin
Oligomycin
Oligomycins are macrolides created by Streptomyces that can be poisonous to other organisms.-Function:They have use as antibiotics.In addition, oligomycin inhibits ATP synthase by blocking its proton channel , which is necessary for oxidative phosphorylation of ADP to ATP . The inhibition of ATP...
, an antibiotic, is able to inhibit the FO unit of ATP synthase.
These functional regions consist of different protein subunits - refer to tables.
F1- ATP Synthase structure
The F1 particle is large and can be seen in the transmission electron microscope by negative staining. These are particles of 9 nm diameter that pepper the inner mitochondrial membrane. They were originally called elementary particles and were thought to contain the entire respiratory apparatus of the mitochondrion, but, through a long series of experiments, Ephraim Racker and his colleagues (who first isolated the F1 particle in 1961) were able to show that this particle is correlated with ATPase activity in uncoupled mitochondria and with the ATPase activity in submitochondrial particleSubmitochondrial particle
A submitochondrial particle is a compartmentalized membranous product of exposing mitochondria to ultrasound. This causes the cristae to pinch off forcing the inner mitochondrial membrane inside out. As a consequence, the F1 particle becomes exposed and on the outside...
s created by exposing mitochondria to ultrasound. This ATPase activity was further associated with the creation of ATP by a long series of experiments in many laboratories.
| Subunit | Human Gene |
|---|---|
| alpha ATP synthase alpha/beta subunits ATPases are membrane-bound enzyme complexes/ion transporters that combine ATP synthesis and/or hydrolysis with the transport of protons across a membrane... |
ATP5A1 ATP5A1 ATP synthase subunit alpha, mitochondrial is an enzyme that in humans is encoded by the ATP5A1 gene.-Further reading:... , ATPAF2 ATPAF2 ATP synthase mitochondrial F1 complex assembly factor 2 is an enzyme that in humans is encoded by the ATPAF2 gene.-Further reading:... |
| beta ATP synthase alpha/beta subunits ATPases are membrane-bound enzyme complexes/ion transporters that combine ATP synthesis and/or hydrolysis with the transport of protons across a membrane... |
ATP5B ATP5B ATP synthase subunit beta, mitochondrial is an enzyme that in humans is encoded by the ATP5B gene.-Further reading:... , ATPAF1 ATPAF1 ATP synthase mitochondrial F1 complex assembly factor 1, also known as ATP11 homolog, is a protein that in humans is encoded by the ATPAF1 gene.- Function :... , C16orf7 C16orf7 Chromosome 16 open reading frame 7 is a protein that in humans is encoded by the C16orf7 gene.... |
| gamma ATP synthase gamma subunit Gamma subunit of ATP synthase F1 complex forms the central shaft that connects the F0 rotary motor to the F1 catalytic core. F-ATP synthases are composed of two linked complexes: the F1 ATPase complex is the catalytic core and is composed of 5 subunits , while the F0 ATPase complex... |
ATP5C1 ATP5C1 ATP synthase subunit gamma, mitochondrial is an enzyme that in humans is encoded by the ATP5C1 gene.-Further reading:... |
| delta ATP synthase delta subunit ATP synthase delta subunit is a subunit of bacterial and chloroplast ATPase, or OSCP in mitochondrial ATPase .... |
ATP5D ATP5D ATP synthase subunit delta, mitochondrial is an enzyme that in humans is encoded by the ATP5D gene.-Further reading:... |
| epsilon ATP synthase right|thumb|300px|Molecular model of ATP synthase by X-ray diffraction methodATP synthase is an important enzyme that provides energy for the cell to use through the synthesis of adenosine triphosphate . ATP is the most commonly used "energy currency" of cells from most organisms... |
ATP5E ATP5E The epsilon subunit is located in the stalk region of the F1 complex, and acts as an inhibitor of the ATPase catalytic core. The epsilon subunit can assume two conformations, contracted and extended, where the latter inhibits ATP hydrolysis. The conformation of the epsilon subunit is determined by... |
FO - ATP Synthase Structure
The FO region of ATP synthase is a proton pore which is emmbeded into the mitochondrial membrane. It consists of three main subunits A, B and C, and (in humans) six additional subunits, dATP5H
ATP synthase subunit d, mitochondrial is an enzyme that in humans is encoded by the ATP5H gene.-Further reading:...
, e
ATP5I
In yeast, the FO complex E subunit appears to play an important role in supporting F-ATPase dimerisation. This subunit is anchored to the inner mitochondrial membrane via its N-terminal region, which is involved in stabilising subunits G and K of the FO complex...
, f
ATP5J2
The ATP5J2 gene encodes the ATP synthase subunit f, mitochondrial enzyme in humans.-Further reading:...
, g
ATP5L
The function of subunit G is currently unknown. There is no counterpart in chloroplast or bacterial F-ATPases identified so far.-Further reading:...
, F6 and 8 (or A6L).
| Subunit | Human Gene |
|---|---|
| A | ATP6 |
| B | ATP5F1 ATP5F1 The B subunits are part of the peripheral stalk that links the F1 and FO complexes together, and which acts as a stator to prevent certain subunits from rotating with the central rotary element. The peripheral stalk differs in subunit composition between mitochondrial, chloroplast and bacterial... |
| C ATP synthase subunit C ATPase, subunit C of F0/V0 complex is the main transmembrane subunit of V-type , A-type and F-type ATP synthases.ATPases are membrane-bound enzyme complexes/ion transporters that combine ATP synthesis and/or hydrolysis with the transport of protons across a membrane... |
ATP5G1 ATP5G1 ATP synthase lipid-binding protein, mitochondrial is an enzyme that in humans is encoded by the ATP5G1 gene.-Further reading:... , ATP5G2 ATP5G2 ATP synthase lipid-binding protein, mitochondrial is an enzyme that in humans is encoded by the ATP5G2 gene.-Further reading:... , ATP5G3 ATP5G3 ATP synthase lipid-binding protein, mitochondrial is an enzyme that in humans is encoded by the ATP5G3 gene.-Further reading:... |
Binding-change model
In the 1960s through the 1970s, Paul BoyerPaul D. Boyer
- External links :* , from the Office of Scientific and Technical Information, United States Department of Energy* * *...
developed the binding change, or flip-flop, mechanism, which postulated that ATP synthesis is coupled with a conformational change in the ATP synthase generated by rotation of the gamma subunit. The research group of John E. Walker
John E. Walker
Professor Sir John Ernest Walker is an English chemist who won the Nobel Prize in Chemistry in 1997. He is currently the director of the MRC Mitochondrial Biology Unit in Cambridge, and a Fellow of Sidney Sussex College.He was born in Halifax, Yorkshire, the son of Thomas Ernest Walker, a...
, then at the MRC Laboratory of Molecular Biology in Cambridge but now at the MRC Mitochondrial Biology Unit (also in Cambridge), crystallized the F1 catalytic-domain of ATP synthase. The structure, at the time the largest asymmetric protein structure known, indicated that Boyer's rotary-catalysis model was, in essence, correct. For elucidating this, Boyer and Walker shared half of the 1997 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
. Jens Christian Skou
Jens Christian Skou
Jens Christian Skou is a Danish chemist and Nobel laureate.Skou was born in Lemvig, Denmark to a wealthy family. His father Magnus Martinus Skou was a timber and coal merchant. His mother Ane-Margrethe Skou took over the company after the death of his father. At the age of 15 Skou entered a...
received the other half of the Chemistry prize that year "for the first discovery of an ion-transporting enzyme, Na+, K+ -ATPase."

Protein subunit
In structural biology, a protein subunit or subunit protein is a single protein molecule that assembles with other protein molecules to form a protein complex: a multimeric or oligomeric protein. Many naturally occurring proteins and enzymes are multimeric...
s (3 of each), arranged like segments of an orange around an asymmetrical gamma subunit. According to the current model of ATP synthesis (known as the alternating catalytic model), the proton-motive force across the inner mitochondrial membrane, generated by the electron transport chain, drives the passage of proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s through the membrane via the FO region of ATP synthase. A portion of the FO (the ring of c-subunits
ATP synthase subunit C
ATPase, subunit C of F0/V0 complex is the main transmembrane subunit of V-type , A-type and F-type ATP synthases.ATPases are membrane-bound enzyme complexes/ion transporters that combine ATP synthesis and/or hydrolysis with the transport of protons across a membrane...
) rotates as the protons pass through the membrane. The c-ring
ATP synthase subunit C
ATPase, subunit C of F0/V0 complex is the main transmembrane subunit of V-type , A-type and F-type ATP synthases.ATPases are membrane-bound enzyme complexes/ion transporters that combine ATP synthesis and/or hydrolysis with the transport of protons across a membrane...
is tightly attached to the asymmetric central stalk (consisting primarily of the gamma subunit), which rotates within the alpha3beta3 of F1 causing the 3 catalytic nucleotide binding sites to go through a series of conformational changes that leads to ATP synthesis. The major F1 subunits are prevented from rotating in sympathy with the central stalk rotor by a peripheral stalk that joins the alpha3beta3 to the non-rotating portion of FO. The structure of the intact ATP synthase is currently known at low-resolution from electron cryo-microscopy (cryo-EM) studies of the complex. The cryo-EM model of ATP synthase suggests that the peripheral stalk is a flexible structure that wraps around the complex as it joins F1 to FO. Under the right conditions, the enzyme reaction can also be carried out in reverse, with ATP hydrolysis driving proton pumping across the membrane.
The binding change mechanism involves the active site of a β subunit cycling between three states. In the "open" state, ADP and phosphate enter the active site, in the diagram to the right this is shown in red. The protein then closes up around the molecules and binds them loosely - the "loose" state (shown in orange). The enzyme then undergoes another change in shape and forces these molecules together, with the active site in the resulting "tight" state (shown in pink) binding the newly-produced ATP molecule with very high affinity
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
. Finally, the active site cycles back to the open state, releasing ATP and binding more ADP and phosphate, ready for the next cycle of ATP production.
Physiological role
Like other enzymes, the activity of F1FO ATP synthase is reversible. Large-enough quantities of ATP cause it to create a transmembrane protonProton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
gradient
Gradient
In vector calculus, the gradient of a scalar field is a vector field that points in the direction of the greatest rate of increase of the scalar field, and whose magnitude is the greatest rate of change....
, this is used by fermenting bacteria that do not have an electron transport chain, and hydrolyze ATP to make a proton gradient, which they use for flagella and transport of nutrients into the cell.
In respiring bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
under physiological conditions, ATP synthase, in general, runs in the opposite direction, creating ATP while using the protonmotive force created by the electron transport chain as a source of energy. The overall process of creating energy in this fashion is termed oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
.
The same process takes place in the mitochondria, where ATP synthase is located in the inner mitochondrial membrane (so that F1-part sticks into mitochondrial matrix, where ATP synthesis takes place).
Evolution of ATP synthase
The evolutionEvolution
Evolution is any change across successive generations in the heritable characteristics of biological populations. Evolutionary processes give rise to diversity at every level of biological organisation, including species, individual organisms and molecules such as DNA and proteins.Life on Earth...
of ATP synthase is thought to be an example of modular evolution, where two subunits with their own functions have become associated and gained new functionality. This coupling must have occurred early in the evolution of life as evidenced by essentially the same structure and processes of ATP synthase enzymes conserved in all kingdoms of life. The F-ATP synthase shows large amounts of similarity both functionally and mechanically to the V-ATPase
V-ATPase
Vacuolar-type H+-ATPase is a highly conserved evolutionarily ancient enzyme with remarkably diverse functions in eukaryotic organisms. V-ATPases acidify a wide array of intracellular organelles and pump protons across the plasma membranes of numerous cell types...
. However whilst the F-ATP synthase generates ATP by utilising a proton gradient the V-ATPase
V-ATPase
Vacuolar-type H+-ATPase is a highly conserved evolutionarily ancient enzyme with remarkably diverse functions in eukaryotic organisms. V-ATPases acidify a wide array of intracellular organelles and pump protons across the plasma membranes of numerous cell types...
is responsible for generating a proton gradient at the expense of ATP, generating pH values as low as 1.
The F1 particle also shows significant similarity to hexameric DNA helicases and the FO particle shows some similarity to H+ powered flagellar motor
Flagellum
A flagellum is a tail-like projection that protrudes from the cell body of certain prokaryotic and eukaryotic cells, and plays the dual role of locomotion and sense organ, being sensitive to chemicals and temperatures outside the cell. There are some notable differences between prokaryotic and...
complexes. The α3β3 hexamer of the F1 particle shows significant structural similarity to hexameric DNA helicases; both form a ring with 3 fold rotational symmetry with a central pore. Both also have roles dependent on the relative rotation of a macromolecule within the pore; the DNA helicases use the helical shape of DNA to drive their motion along the DNA molecule and to detect supercoiling whilst the α3β3 hexamer uses the conformational changes due rotation of the γ subunit to drive an enzymatic reaction.
The H+ motor of the FO particle shows great functional similarity to the H+ motors seen in flagellar motors. Both feature a ring of many small alpha helical proteins that rotate relative to nearby stationary proteins using a H+ potential gradient as an energy source. This is, however, a fairly tenuous link - the overall structure of flagellar motors is far more complex than the FO particle and the ring of rotating proteins is far larger, with around 30 compared to the 10, 11, or 14 known in the FO complex.
The modular evolution theory for the origin of ATP synthase suggests that two subunits with independent function, a DNA helicase with ATPase activity and a H+ motor, were able to bind, and the rotation of the motor drive the ATPase activity of the helicase in reverse. This would then evolve to become more efficient, and eventually develop into the complex ATP synthases seen today. In alternative fashion, the DNA helicase/H+ motor complex may have had H+ pump activity, the ATPase activity of the helicase driving the H+ motor in reverse. This could later evolve to carry out the reverse reaction and act as an ATP synthase.
Human ATP synthase
The following is a list of humans genes that encode components of ATP synthases:- ATP5A1, ATP5AL1
- ATP5BATP5BATP synthase subunit beta, mitochondrial is an enzyme that in humans is encoded by the ATP5B gene.-Further reading:...
, ATP5BL1 - ATP5C2, ATP5DATP5DATP synthase subunit delta, mitochondrial is an enzyme that in humans is encoded by the ATP5D gene.-Further reading:...
, ATP5EATP5EThe epsilon subunit is located in the stalk region of the F1 complex, and acts as an inhibitor of the ATPase catalytic core. The epsilon subunit can assume two conformations, contracted and extended, where the latter inhibits ATP hydrolysis. The conformation of the epsilon subunit is determined by...
, ATP5F1ATP5F1The B subunits are part of the peripheral stalk that links the F1 and FO complexes together, and which acts as a stator to prevent certain subunits from rotating with the central rotary element. The peripheral stalk differs in subunit composition between mitochondrial, chloroplast and bacterial...
, ATP5G1ATP5G1ATP synthase lipid-binding protein, mitochondrial is an enzyme that in humans is encoded by the ATP5G1 gene.-Further reading:...
, ATP5G2ATP5G2ATP synthase lipid-binding protein, mitochondrial is an enzyme that in humans is encoded by the ATP5G2 gene.-Further reading:...
, ATP5G3ATP5G3ATP synthase lipid-binding protein, mitochondrial is an enzyme that in humans is encoded by the ATP5G3 gene.-Further reading:...
, ATP5HATP5HATP synthase subunit d, mitochondrial is an enzyme that in humans is encoded by the ATP5H gene.-Further reading:...
, ATP5HP1, ATP5IATP5IIn yeast, the FO complex E subunit appears to play an important role in supporting F-ATPase dimerisation. This subunit is anchored to the inner mitochondrial membrane via its N-terminal region, which is involved in stabilising subunits G and K of the FO complex...
, ATP5JATP5JThe F6 subunit is part of the peripheral stalk that links the F1 and FO complexes together, and which acts as a stator to prevent certain subunits from rotating with the central rotary element. The peripheral stalk differs in subunit composition between mitochondrial, chloroplast and bacterial...
, ATP5J2ATP5J2The ATP5J2 gene encodes the ATP synthase subunit f, mitochondrial enzyme in humans.-Further reading:...
, ATP5LATP5LThe function of subunit G is currently unknown. There is no counterpart in chloroplast or bacterial F-ATPases identified so far.-Further reading:...
, ATP5L2, ATP5OATP5OATP synthase subunit O, mitochondrial is an enzyme that in humans is encoded by the ATP5O gene.-Further reading:...
, ATP5SATP5SATP synthase subunit s, mitochondrial is an enzyme that in humans is encoded by the ATP5S gene.-Further reading:... - ATP6ATP synthase chain AATP synthase F0 subunit 6 is a subunit of F0 complex of transmembrane F-type ATP synthase.- Function :...
, ATP6AP1ATP6AP1V-type proton ATPase subunit S1 is an enzyme that in humans is encoded by the ATP6AP1 gene.-Further reading:...
, ATP6AP2ATP6AP2The renin receptor also known as ATPase H-transporting lysosomal accessory protein 2, or the prorenin receptor, is a protein that in humans is encoded by the ATP6AP2 gene.- Function :The renin receptor binds renin and prorenin... - ATPSBL1, ATPSBL2
- MT-ATP6, MT-ATP8
Plant ATP synthase
In plants, ATP synthase is also present in chloroplasts (CF1FO-ATP synthase). The enzyme is integrated into thylakoidThylakoid
A thylakoid is a membrane-bound compartment inside chloroplasts and cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a thylakoid membrane surrounding a thylakoid lumen. Chloroplast thylakoids frequently form stacks of disks referred to as...
membrane; the CF1-part sticks into stroma
Stroma (fluid)
Stroma, in botany, refers to the colourless fluid surrounding the grana within the Plastid, chloroplast.Within the stroma are grana, stacks of thylakoids, the sub-organelles, where photosynthesis is commenced before the chemical changes are completed in the stroma.Photosynthesis occurs in two...
, where dark reactions of photosynthesis (Also called the light-independent reactions or the Calvin cycle
Calvin cycle
The Calvin cycle or Calvin–Benson-Bassham cycle or reductive pentose phosphate cycle or C3 cycle or CBB cycle is a series of biochemical redox reactions that take place in the stroma of chloroplasts in photosynthetic organisms...
) and ATP synthesis take place. The overall structure and the catalytic mechanism of the chloroplast ATP synthase are almost the same as those of the mitochondrial enzyme. However, in chloroplasts, the proton motive force is generated not by respiratory electron transport chain but by primary photosynthetic proteins.
Bovine ATP synthase
The ATP synthase isolated from bovine heart mitochondria (Bos taurus) is, in terms of biochemistry and structure, the best-characterized ATP synthase. Beef heart is used as a source for the enyzme because of the high concentration of mitochondria in cardiac muscle.E. coli ATP synthase
E. coli ATP synthase is the simplest known form of ATP synthase, with 8 different subunit types.Yeast ATP synthase
Yeast ATP synthase is one of the best-studied eukaryotic ATP synthases; and five F1, eight FO subunits, and seven associated proteins have been identified. Most of these proteins have homologues in other eukaryotes.Subunits of ATP synthase
- ATP synthase alpha/beta subunitsATP synthase alpha/beta subunitsATPases are membrane-bound enzyme complexes/ion transporters that combine ATP synthesis and/or hydrolysis with the transport of protons across a membrane...
- ATP synthase delta subunitATP synthase delta subunitATP synthase delta subunit is a subunit of bacterial and chloroplast ATPase, or OSCP in mitochondrial ATPase ....
- ATP synthase gamma subunitATP synthase gamma subunitGamma subunit of ATP synthase F1 complex forms the central shaft that connects the F0 rotary motor to the F1 catalytic core. F-ATP synthases are composed of two linked complexes: the F1 ATPase complex is the catalytic core and is composed of 5 subunits , while the F0 ATPase complex...
- ATP synthase subunit CATP synthase subunit CATPase, subunit C of F0/V0 complex is the main transmembrane subunit of V-type , A-type and F-type ATP synthases.ATPases are membrane-bound enzyme complexes/ion transporters that combine ATP synthesis and/or hydrolysis with the transport of protons across a membrane...
Other
- ATP10 proteinATP10 proteinIn molecular biology, ATP10 protein is an ATP synthase assembly factor. It is essential for the assembly of the mitochondrial F1-F0 complex. A yeast nuclear gene encodes a product that is essential for the assembly of a functional mitochondrial ATPase complex...
required for the assembly of the FO sector of the mitochondrial ATPase complex. - V-ATPaseV-ATPaseVacuolar-type H+-ATPase is a highly conserved evolutionarily ancient enzyme with remarkably diverse functions in eukaryotic organisms. V-ATPases acidify a wide array of intracellular organelles and pump protons across the plasma membranes of numerous cell types...
- Oxidative phosphorylationOxidative phosphorylationOxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
- MitochondrionMitochondrionIn cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
- ChloroplastChloroplastChloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
- Electron transfer chain
- Proton pumpProton pumpA proton pump is an integral membrane protein that is capable of moving protons across a cell membrane, mitochondrion, or other organelle. Mechanisms are based on conformational changes of the protein structure or on the Q cycle.-Function:...
- Transmembrane ATPase
- FlavoproteinFlavoproteinFlavoproteins are proteins that contain a nucleic acid derivative of riboflavin: the flavin adenine dinucleotide or flavin mononucleotide ....
- P-ATPase
- Rotating locomotion in living systems
External links
- "ATP synthase - a splendid molecular machine"
- Well illustrated ATP synthase lecture by Antony Crofts of the University of Illinois at Urbana-ChampaignUniversity of Illinois at Urbana-ChampaignThe University of Illinois at Urbana–Champaign is a large public research-intensive university in the state of Illinois, United States. It is the flagship campus of the University of Illinois system...
. - Proton and Sodium translocating F-type, V-type and A-type ATPases in OPM database
- The Nobel Prize in Chemistry 1997 to Paul D. Boyer and John E. Walker for the enzymatic mechanism of synthesis of ATP; and to Jens C. Skou, for discovery of an ion-transporting enzyme, Na+, K+-ATPase.
- Harvard Multimedia Production Site - Videos - ATP synthesis animation

