
Nicotinamide adenine dinucleotide
Encyclopedia
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cell
s. The compound is a dinucleotide, since it consists of two nucleotide
s joined through their phosphate groups. One nucleotide contains an adenine
base and the other nicotinamide
.
In metabolism
, NAD is involved in redox
reactions, carrying electron
s from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD is an oxidizing agent
– it accepts electrons from other molecules and becomes reduced
. This reaction forms NADH, which can then be used as a reducing agent
to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate
of enzymes that add or remove chemical groups
from proteins, in posttranslational modification
s. Because of the importance of these functions, the enzyme
s involved in NAD metabolism are targets for drug discovery
.
In organisms, NAD can be synthesized from simple building-blocks (de novo
) from the amino acids tryptophan
or aspartic acid
. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin
called niacin
. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate
(NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.
s joined by a pair of bridging phosphate groups. The nucleotide
s consist of ribose
rings, one with adenine
attached to the first carbon atom (the 1'
position) and the other with nicotinamide
at this position. The nicotinamide moiety can be attached in two orientations to this anomer
ic carbon atom. Because of these two possible structures, the compound exists as two diastereomer
s. It is the β-nicotinamide diastereomer of NAD that is found in organisms. These nucleotides are joined together by a bridge of two phosphate
groups through the 5' carbons.
 In metabolism, the compound accepts or donates electrons in redox reactions. Such reactions (summarized in formula below) involve the removal of two hydrogen atoms from the reactant (R), in the form of a hydride ion
In metabolism, the compound accepts or donates electrons in redox reactions. Such reactions (summarized in formula below) involve the removal of two hydrogen atoms from the reactant (R), in the form of a hydride ion
(H−), and a proton
(H). The proton is released into solution, while the reductant RH is oxidized and NAD reduced to NADH by transfer of the hydride to the nicotinamide ring.
From the hydride electron pair, one electron is transferred to the positively charged nitrogen of the nicotinamide ring of NAD, and the second hydrogen atom transferred to the C4 carbon atom opposite this nitrogen. The midpoint potential of the NAD/NADH redox pair is −0.32 volt
s, which makes NADH a strong reducing agent. The reaction is easily reversible, when NADH reduces another molecule and is re-oxidized to NAD. This means the coenzyme can continuously cycle between the NAD and NADH forms without being consumed.
In appearance, all forms of this coenzyme are white amorphous
powders that are hygroscopic
and highly water-soluble. The solids are stable if stored dry and in the dark. Solutions of NAD are colorless and stable for about a week at 4 °C
and neutral pH
, but decompose rapidly in acids or alkalis. Upon decomposition, they form products that are enzyme inhibitor
s.
Both NAD and NADH strongly absorb ultraviolet
light because of the adenine. For example, peak absorption of NAD is at a wavelength
of 259 nanometers (nm), with an extinction coefficient
of 16,900 M−1cm
−1. NADH also absorbs at higher wavelengths, with a second peak in UV absorption at 339 nm with an extinction coefficient of 6,220 M−1cm−1. This difference in the ultraviolet absorption spectra between the oxidized and reduced forms of the coenzymes at higher wavelengths makes it simple to measure the conversion of one to another in enzyme assay
s – by measuring the amount of UV absorption at 340 nm using a spectrophotometer
.
NAD and NADH also differ in their fluorescence
. NADH in solution has an emission peak at 460 nm and a fluorescence lifetime of 0.4 nanosecond
s, while the oxidized form of the coenzyme does not fluoresce. The properties of the fluorescence signal changes when NADH binds to proteins, so these changes can be used to measure dissociation constant
s, which are useful in the study of enzyme kinetics
. These changes in fluorescence are also used to measure changes in the redox state of living cells, through fluorescence microscopy
.
of wet weight, about 10 times the concentration of NADP and NADPH in the same cells. The actual concentration of NAD in cell cytosol
is harder to measure, with recent estimates in animal cells, ranging around 0.3 mM, and approximately 1.0 to 2.0 mM in yeast
. However, more than 80% of mitochondrial NADH is bound to proteins, so the concentration in solution is much lower.
Data for other compartments in the cell are limited, although, in the mitochondrion
the concentration of NAD is similar to that in the cytosol. This NAD is carried into the mitochondrion by a specific membrane transport protein, since the coenzyme cannot diffuse
across membranes.
The balance between the oxidized and reduced forms of nicotinamide adenine dinucleotide is called the NAD/NADH ratio. This ratio is an important component of what is called the redox state of a cell, a measurement that reflects both the metabolic activities and the health of cells. The effects of the NAD/NADH ratio are complex, controlling the activity of several key enzymes, including glyceraldehyde 3-phosphate dehydrogenase
and pyruvate dehydrogenase
. In healthy mammalian tissues, estimates of the ratio between free NAD and NADH in the cytoplasm typically lie around 700; the ratio is thus favourable for oxidative reactions. The ratio of total NAD/NADH is much lower, with estimates ranging from 0.05 to 4. In contrast, the NADP/NADPH
ratio is normally about 0.005, so NADPH is the dominant form of this coenzyme. These different ratios are key to the different metabolic roles of NADH and NADPH.
pathway from amino acid
s or in salvage pathways by recycling preformed components such as nicotinamide
back to NAD.
(QA) from an amino acid—either tryptophan
(Trp) in animals and some bacteria, or aspartic acid
in some bacteria and plants. The quinolinic acid is converted to nicotinic acid mononucleotide (NaMN) by transfer of a phosphoribose moiety. An adenylate moiety is then transferred to form nicotinic acid adenine dinucleotide (NaAD). Finally, the nicotinic acid moiety in NaAD is amidated
to a nicotinamide (Nam) moiety, forming nicotinamide adenine dinucleotide.
In a further step, some NAD is converted into NADP by NAD+ kinase
, which phosphorylates NAD. In most organisms, this enzyme uses ATP as the source of the phosphate group, although several bacteria such as Mycobacterium tuberculosis
and a hyperthermophilic archaeon Pyrococcus horikoshii
, use inorganic polyphosphate
as an alternative phosphoryl donor.
(NR). These compounds can be taken up from the diet, where the mixture of nicotinic acid and nicotinamide are called vitamin B or niacin
. However, these compounds are also produced within cells, when the nicotinamide moiety is released from NAD in ADP-ribose transfer reactions. Indeed, the enzymes involved in these salvage pathways appear to be concentrated in the cell nucleus
, which may compensate for the high level of reactions that consume NAD in this organelle
. Cells can also take up extracellular NAD from their surroundings.
Despite the presence of the de novo pathway, the salvage reactions are essential in humans; a lack of niacin in the diet causes the vitamin deficiency disease pellagra
. This high requirement for NAD results from the constant consumption of the coenzyme in reactions such as posttranslational modifications, since the cycling of NAD between oxidized and reduced forms in redox reactions does not change the overall levels of the coenzyme.
The salvage pathways used in microorganism
s differ from those of mammal
s. Some pathogens, such as the yeast Candida glabrata
and the bacterium Haemophilus influenzae
are NAD auxotrophs – they cannot synthesize NAD – but possess salvage pathways and thus are dependent on external sources of NAD or its precursors. Even more surprising is the intracellular pathogen
Chlamydia trachomatis
, which lacks recognizable candidates for any genes involved in the biosynthesis or salvage of both NAD and NADP, and must acquire these coenzymes from its host
.
. It acts as a coenzyme in redox
reactions, as a donor of ADP-ribose moieties in ADP-ribosylation
reactions, as a precursor of the second messenger
molecule cyclic ADP-ribose
, as well as acting as a substrate for bacterial DNA ligase
s and a group of enzymes called sirtuin
s that use NAD to remove acetyl groups
from proteins.
s. The correct names for these enzymes contain the names of both their substrates: for example NADH-ubiquinone oxidoreductase
catalyzes the oxidation of NADH by coenzyme Q
. However, these enzymes are also referred to as dehydrogenases or reductases, with NADH-ubiquinone oxidoreductase commonly being called NADH dehydrogenase or sometimes coenzyme Q reductase.
When bound to a protein, NAD and NADH are usually held within a structural motif
known as the Rossmann fold
. The motif is named after Michael Rossmann who was the first scientist to notice how common this structure is within nucleotide-binding proteins. This fold contains three or more parallel beta strands
linked by two alpha helices
in the order beta-alpha-beta-alpha-beta. This forms a beta sheet flanked by a layer of alpha helices on each side. Because each Rossmann fold binds one nucleotide, binding domains for the dinucleotide NAD consist of two paired Rossmann folds, with each fold binding one nucleotide within the cofactor. However, this fold is not universal among NAD-dependent enzymes, since a class of bacterial enzymes involved in amino acid
metabolism have recently been discovered that bind the coenzyme, but lack this motif.
When bound in the active site of an oxidoreductase, the nicotinamide ring of the coenzyme is positioned so that it can accept a hydride from the other substrate. Since the C4 carbon that accepts the hydrogen is prochiral
, this can be exploited in enzyme kinetics
to give information about the enzyme's mechanism. This is done by mixing an enzyme with a substrate that has deuterium
atoms substituted for the hydrogens, so the enzyme will reduce NAD by transferring deuterium rather than hydrogen. In this case, an enzyme can produce one of two stereoisomer
s of NADH. In some enzymes the hydrogen is transferred from above the plane of the nicotinamide ring; these are called class A oxidoreductases, whereas class B enzymes transfer the atom from below.
Despite the similarity in how proteins bind the two coenzymes, enzymes almost always show a high level of specificity for either NAD or NADP. This specificity reflects the distinct metabolic roles of the respective coenzymes, and is the result of distinct sets of amino acid
residues in the two types of coenzyme-binding pocket. For instance, in the active site of NADP-dependent enzymes, an ionic bond
is formed between a basic amino acid side-chain and the acidic phosphate group of NADP. On the converse, in NAD-dependent enzymes the charge in this pocket is reversed, preventing NADP from binding. However, there are a few exceptions to this general rule, and enzymes such as aldose reductase
, glucose-6-phosphate dehydrogenase
, and methylenetetrahydrofolate reductase
can use both coenzymes in some species.
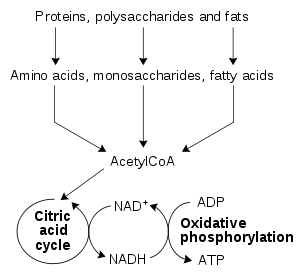
The redox reactions catalyzed by oxidoreductases are vital in all parts of metabolism, but one particularly important area where these reactions occur is in the release of energy from nutrients. Here, reduced compounds such as glucose
and fatty acids are oxidized, thereby releasing energy. This energy is transferred to NAD by reduction to NADH, as part of beta oxidation
, glycolysis
, and the citric acid cycle
. In eukaryote
s the electrons carried by the NADH that is produced in the cytoplasm
are transferred into the mitochondrion
(to reduce mitochondrial NAD) by mitochondrial shuttle
s, such as the malate-aspartate shuttle
. The mitochondrial NADH is then oxidized in turn by the electron transport chain
, which pumps protons across a membrane and generates ATP through oxidative phosphorylation
. These shuttle systems also have the same transport function in chloroplast
s.
Since both the oxidized and reduced forms of nicotinamide adenine dinucleotide are used in these linked sets of reactions, the cell maintains significant concentrations of both NAD and NADH, with the high NAD/NADH ratio allowing this coenzyme to act as both an oxidizing and a reducing agent. In contrast, the main function of NADPH is as a reducing agent in anabolism
, with this coenzyme being involved in pathways such as fatty acid synthesis
and photosynthesis
. Since NADPH is needed to drive redox reactions as a strong reducing agent, the NADP/NADPH ratio is kept very low.
Although it is important in catabolism, NADH is also used in anabolic reactions, such as gluconeogenesis
. This need for NADH in anabolism poses a problem for prokaryotes growing on nutrients that release only a small amount of energy. For example, nitrifying
bacteria such as Nitrobacter
oxidize nitrite to nitrate, which releases sufficient energy to pump protons and generate ATP, but not enough to produce NADH directly. As NADH is still needed for anabolic reactions, these bacteria use a nitrite oxidoreductase
to produce enough proton-motive force
to run part of the electron transport chain in reverse, generating NADH.
add the ADP-ribose moiety of this molecule to proteins, in a posttranslational modification
called ADP-ribosylation
. NAD may also be added onto cellular RNA
as a base
modification. ADP-ribosylation involves either the addition of a single ADP-ribose moiety, in mono-ADP-ribosylation, or the transferral of ADP-ribose to proteins in long branched chains, which is called poly(ADP-ribosyl)ation. Mono-ADP-ribosylation was first identified as the mechanism of a group of bacterial toxin
s, notably cholera toxin
, but it is also involved in normal cell signaling
. Poly(ADP-ribosyl)ation is carried out by the poly(ADP-ribose) polymerase
s. The poly(ADP-ribose) structure is involved in the regulation of several cellular events and is most important in the cell nucleus
, in processes such as DNA repair
and telomere
maintenance. In addition to these functions within the cell, a group of extracellular
ADP-ribosyltransferases has recently been discovered, but their functions remain obscure.
Another function of this coenzyme in cell signaling is as a precursor of cyclic ADP-ribose
, which is produced from NAD by ADP-ribosyl cyclases, as part of a second messenger system
. This molecule acts in calcium signaling
by releasing calcium from intracellular stores. It does this by binding to and opening a class of calcium channels called ryanodine receptor
s, which are located in the membranes of organelle
s, such as the endoplasmic reticulum
.
NAD is also consumed by sirtuin
s, which are NAD-dependent deacetylases
, such as Sir2
. These enzymes act by transferring an acetyl
group from their substrate protein to the ADP-ribose moiety of NAD; this cleaves the coenzyme and releases nicotinamide and O-acetyl-ADP-ribose. The sirtuins mainly seem to be involved in regulating transcription
through deacetylating histones and altering nucleosome
structure. However, non-histone proteins can be deacetylated by sirtuins as well. These activities of sirtuins are particularly interesting because of their importance in the regulation of aging.
Other NAD-dependent enzymes include bacterial DNA ligase
s, which join two DNA ends by using NAD as a substrate to donate an adenosine monophosphate
(AMP) moiety to the 5' phosphate of one DNA end. This intermediate is then attacked by the 3' hydroxyl group of the other DNA end, forming a new phosphodiester bond
. This contrasts with eukaryotic DNA ligases, which use ATP
to form the DNA-AMP intermediate.
and the research into future treatments for disease. Drug design
and drug development exploits NAD in three ways: as a direct target of drugs, by designing enzyme inhibitor
s or activators based on its structure that change the activity of NAD-dependent enzymes, and by trying to inhibit NAD biosynthesis.
The coenzyme NAD is not itself currently used as a treatment for any disease. However, it is potentially useful in the therapy of neurodegenerative diseases such as Alzheimer's
and Parkinson disease. Evidence on the use of NAD in neurodegeneration
is mixed; studies in mice
are promising, whereas a placebo-controlled clinical trial failed to show any effect. NAD is also a direct target of the drug isoniazid
, which is used in the treatment of tuberculosis
, an infection caused by Mycobacterium tuberculosis
. Isoniazid is a prodrug
and once it has entered the bacteria, it is activated by a peroxidase
, which oxidizes the compound into a free radical form. This radical then reacts with NADH, to produce adducts that are very potent inhibitors of the enzymes enoyl-acyl carrier protein reductase
, and dihydrofolate reductase
.
Since a large number of oxidoreductases use NAD and NADH as substrates, and bind them using a highly conserved structural motif, the idea that inhibitors based on NAD could be specific to one enzyme is surprising. However, this can be possible: for example, inhibitors based on the compounds mycophenolic acid
and tiazofurin
inhibit IMP dehydrogenase
at the NAD binding site. Because of the importance of this enzyme in purine metabolism
, these compounds may be useful as anti-cancer, anti-viral, or immunosuppressive drug
s. Other drugs are not enzyme inhibitors, but instead activate enzymes involved in NAD metabolism. Sirtuin
s are a particularly interesting target for such drugs, since activation of these NAD-dependent deacetylases extends lifespan. Compounds such as resveratrol
increase the activity of these enzymes, which may be important in their ability to delay aging in both vertebrate, and invertebrate model organism
s.
Because of the differences in the metabolic pathway
s of NAD biosynthesis between organisms, such as between bacteria and humans, this area of metabolism is a promising area for the development of new antibiotic
s. For example, the enzyme nicotinamidase
, which converts nicotinamide to nicotinic acid, is a target for drug design, as this enzyme is absent in humans but present in yeast and bacteria.
biochemists Arthur Harden
and William Youndin in 1906. They noticed that adding boiled and filtered yeast
extract greatly accelerated alcoholic fermentation in unboiled yeast extracts. They called the unidentified factor responsible for this effect a coferment. Through a long and difficult purification from yeast extracts, this heat-stable factor was identified as a nucleotide
sugar phosphate by Hans von Euler-Chelpin
. In 1936, the German
scientist Otto Heinrich Warburg
showed the function of the nucleotide coenzyme in hydride transfer and identified the nicotinamide portion as the site of redox reactions.
A source of nicotinamide was identified in 1938, when Conrad Elvehjem
purified niacin
from liver and showed this vitamin contained nicotinic acid and nicotinamide. Then, in 1939, he provided the first strong evidence that niacin was used to synthesize NAD. In the early 1940s, Arthur Kornberg
made another important contribution towards understanding NAD metabolism, by being the first to detect an enzyme in the biosynthetic pathway. Subsequently, in 1949, the American
biochemists Morris Friedkin and Albert L. Lehninger
proved that NADH linked metabolic pathways such as the citric acid cycle with the synthesis of ATP in oxidative phosphorylation. Finally, in 1959, Jack Preiss and Philip Handler discovered the intermediates and enzymes involved in the biosynthesis of NAD; consequently, de novo synthesis is often called the Preiss-Handler pathway in their honor.
The non-redox roles of NAD(P) are a recent discovery. The first of these functions to be identified was the use of NAD as the ADP-ribose donor in ADP-ribosylation reactions, observed in the early 1960s. Later studies in the 1980s and 1990s revealed the activities of NAD and NADP metabolites in cell signaling – such as the action of cyclic ADP-ribose
, which was discovered in 1987. The metabolism of NAD has remained an area of intense research into the 21st century, with interest being heightened after the discovery of the NAD-dependent protein deacetylases called sirtuin
s in 2000, by Shinichiro Imai and coworkers at the Massachusetts Institute of Technology
.
History, A history of early enzymology., A textbook from the 19th century.
Cell (biology)
The cell is the basic structural and functional unit of all known living organisms. It is the smallest unit of life that is classified as a living thing, and is often called the building block of life. The Alberts text discusses how the "cellular building blocks" move to shape developing embryos....
s. The compound is a dinucleotide, since it consists of two nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
s joined through their phosphate groups. One nucleotide contains an adenine
Adenine
Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
base and the other nicotinamide
Nicotinamide
Nicotinamide, also known as niacinamide and nicotinic acid amide, is the amide of nicotinic acid . Nicotinamide is a water-soluble vitamin and is part of the vitamin B group...
.
In metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
, NAD is involved in redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions, carrying electron
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s from one reaction to another. The coenzyme is, therefore, found in two forms in cells: NAD is an oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
– it accepts electrons from other molecules and becomes reduced
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
. This reaction forms NADH, which can then be used as a reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, the most notable one being a substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
of enzymes that add or remove chemical groups
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
from proteins, in posttranslational modification
Posttranslational modification
Posttranslational modification is the chemical modification of a protein after its translation. It is one of the later steps in protein biosynthesis, and thus gene expression, for many proteins....
s. Because of the importance of these functions, the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
s involved in NAD metabolism are targets for drug discovery
Drug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which drugs are discovered or designed.In the past most drugs have been discovered either by identifying the active ingredient from traditional remedies or by serendipitous discovery...
.
In organisms, NAD can be synthesized from simple building-blocks (de novo
De novo synthesis
De novo synthesis refers to the synthesis of complex molecules from simple molecules such as sugars or amino acids, as opposed to their being recycled after partial degradation. For example, nucleotides are not needed in the diet as they can be constructed from small precursor molecules such as...
) from the amino acids tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
or aspartic acid
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
. In an alternative fashion, more complex components of the coenzymes are taken up from food as the vitamin
Vitamin
A vitamin is an organic compound required as a nutrient in tiny amounts by an organism. In other words, an organic chemical compound is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on...
called niacin
Niacin
"Niacin" redirects here. For the neo-fusion band, see Niacin .Niacin is an organic compound with the formula and, depending on the definition used, one of the forty to eighty essential human nutrients.Niacin is one of five vitamins associated with a pandemic deficiency disease: niacin deficiency...
. Similar compounds are released by reactions that break down the structure of NAD. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD is also converted into nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
(NADP); the chemistry of this related coenzyme is similar to that of NAD, but it has different roles in metabolism.
Physical and chemical properties
Nicotinamide adenine dinucleotide, like all dinucleotides, consists of two nucleotideNucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
s joined by a pair of bridging phosphate groups. The nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
s consist of ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
rings, one with adenine
Adenine
Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA...
attached to the first carbon atom (the 1'
Nucleic acid nomenclature
Molecular biologists use several shorthand terms when referring to nucleic acid molecules, such as DNA and RNA, collectively referred to as nucleic acid nomenclature....
position) and the other with nicotinamide
Nicotinamide
Nicotinamide, also known as niacinamide and nicotinic acid amide, is the amide of nicotinic acid . Nicotinamide is a water-soluble vitamin and is part of the vitamin B group...
at this position. The nicotinamide moiety can be attached in two orientations to this anomer
Anomer
In carbohydrate chemistry, an anomer is a special type of epimer. It is one of two stereoisomers of a cyclic saccharide that differs only in its configuration at the hemiacetal or hemiketal carbon, also called the anomeric carbon. Anomerization is the process of conversion of one anomer to the other...
ic carbon atom. Because of these two possible structures, the compound exists as two diastereomer
Diastereomer
Diastereomers are stereoisomers that are not enantiomers.Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more of the equivalent stereocenters and are not mirror images of each other.When two diastereoisomers differ from each other at...
s. It is the β-nicotinamide diastereomer of NAD that is found in organisms. These nucleotides are joined together by a bridge of two phosphate
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
groups through the 5' carbons.

Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
(H−), and a proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
(H). The proton is released into solution, while the reductant RH is oxidized and NAD reduced to NADH by transfer of the hydride to the nicotinamide ring.
- RH + NAD → NADH + H + R
From the hydride electron pair, one electron is transferred to the positively charged nitrogen of the nicotinamide ring of NAD, and the second hydrogen atom transferred to the C4 carbon atom opposite this nitrogen. The midpoint potential of the NAD/NADH redox pair is −0.32 volt
Volt
The volt is the SI derived unit for electric potential, electric potential difference, and electromotive force. The volt is named in honor of the Italian physicist Alessandro Volta , who invented the voltaic pile, possibly the first chemical battery.- Definition :A single volt is defined as the...
s, which makes NADH a strong reducing agent. The reaction is easily reversible, when NADH reduces another molecule and is re-oxidized to NAD. This means the coenzyme can continuously cycle between the NAD and NADH forms without being consumed.
In appearance, all forms of this coenzyme are white amorphous
Amorphous solid
In condensed matter physics, an amorphous or non-crystalline solid is a solid that lacks the long-range order characteristic of a crystal....
powders that are hygroscopic
Hygroscopy
Hygroscopy is the ability of a substance to attract and hold water molecules from the surrounding environment. This is achieved through either absorption or adsorption with the absorbing or adsorbing material becoming physically 'changed,' somewhat, by an increase in volume, stickiness, or other...
and highly water-soluble. The solids are stable if stored dry and in the dark. Solutions of NAD are colorless and stable for about a week at 4 °C
Celsius
Celsius is a scale and unit of measurement for temperature. It is named after the Swedish astronomer Anders Celsius , who developed a similar temperature scale two years before his death...
and neutral pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
, but decompose rapidly in acids or alkalis. Upon decomposition, they form products that are enzyme inhibitor
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
s.
Both NAD and NADH strongly absorb ultraviolet
Ultraviolet
Ultraviolet light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than X-rays, in the range 10 nm to 400 nm, and energies from 3 eV to 124 eV...
light because of the adenine. For example, peak absorption of NAD is at a wavelength
Wavelength
In physics, the wavelength of a sinusoidal wave is the spatial period of the wave—the distance over which the wave's shape repeats.It is usually determined by considering the distance between consecutive corresponding points of the same phase, such as crests, troughs, or zero crossings, and is a...
of 259 nanometers (nm), with an extinction coefficient
Molar absorptivity
The molar absorption coefficient, molar extinction coefficient, or molar absorptivity, is a measurement of how strongly a chemical species absorbs light at a given wavelength...
of 16,900 M−1cm
Centimetre
A centimetre is a unit of length in the metric system, equal to one hundredth of a metre, which is the SI base unit of length. Centi is the SI prefix for a factor of . Hence a centimetre can be written as or — meaning or respectively...
−1. NADH also absorbs at higher wavelengths, with a second peak in UV absorption at 339 nm with an extinction coefficient of 6,220 M−1cm−1. This difference in the ultraviolet absorption spectra between the oxidized and reduced forms of the coenzymes at higher wavelengths makes it simple to measure the conversion of one to another in enzyme assay
Enzyme assay
Enzyme assays are laboratory methods for measuring enzymatic activity. They are vital for the study of enzyme kinetics and enzyme inhibition.-Enzyme units:...
s – by measuring the amount of UV absorption at 340 nm using a spectrophotometer
Spectrophotometry
In chemistry, spectrophotometry is the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength...
.
NAD and NADH also differ in their fluorescence
Fluorescence
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. It is a form of luminescence. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation...
. NADH in solution has an emission peak at 460 nm and a fluorescence lifetime of 0.4 nanosecond
Nanosecond
A nanosecond is one billionth of a second . One nanosecond is to one second as one second is to 31.7 years.The word nanosecond is formed by the prefix nano and the unit second. Its symbol is ns....
s, while the oxidized form of the coenzyme does not fluoresce. The properties of the fluorescence signal changes when NADH binds to proteins, so these changes can be used to measure dissociation constant
Dissociation constant
In chemistry, biochemistry, and pharmacology, a dissociation constant is a specific type of equilibrium constant that measures the propensity of a larger object to separate reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into...
s, which are useful in the study of enzyme kinetics
Enzyme kinetics
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated...
. These changes in fluorescence are also used to measure changes in the redox state of living cells, through fluorescence microscopy
Fluorescence microscope
A fluorescence microscope is an optical microscope used to study properties of organic or inorganic substances using the phenomena of fluorescence and phosphorescence instead of, or in addition to, reflection and absorption...
.
Concentration and state in cells
In rat liver, the total amount of NAD and NADH is approximately 1 μmole per gramGram
The gram is a metric system unit of mass....
of wet weight, about 10 times the concentration of NADP and NADPH in the same cells. The actual concentration of NAD in cell cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
is harder to measure, with recent estimates in animal cells, ranging around 0.3 mM, and approximately 1.0 to 2.0 mM in yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
. However, more than 80% of mitochondrial NADH is bound to proteins, so the concentration in solution is much lower.
Data for other compartments in the cell are limited, although, in the mitochondrion
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
the concentration of NAD is similar to that in the cytosol. This NAD is carried into the mitochondrion by a specific membrane transport protein, since the coenzyme cannot diffuse
Diffusion
Molecular diffusion, often called simply diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size of the particles...
across membranes.
The balance between the oxidized and reduced forms of nicotinamide adenine dinucleotide is called the NAD/NADH ratio. This ratio is an important component of what is called the redox state of a cell, a measurement that reflects both the metabolic activities and the health of cells. The effects of the NAD/NADH ratio are complex, controlling the activity of several key enzymes, including glyceraldehyde 3-phosphate dehydrogenase
Glyceraldehyde 3-phosphate dehydrogenase
Glyceraldehyde 3-phosphate dehydrogenase is an enzyme of ~37kDa that catalyzes the sixth step of glycolysis and thus serves to break down glucose for energy and carbon molecules...
and pyruvate dehydrogenase
Pyruvate dehydrogenase
Pyruvate dehydrogenase complex is a complex of three enzymes that transform pyruvate into acetyl-CoA by a process called pyruvate decarboxylation. Acetyl-CoA may then be used in the citric acid cycle to carry out cellular respiration, and this complex links the glycolysis metabolic pathway to the...
. In healthy mammalian tissues, estimates of the ratio between free NAD and NADH in the cytoplasm typically lie around 700; the ratio is thus favourable for oxidative reactions. The ratio of total NAD/NADH is much lower, with estimates ranging from 0.05 to 4. In contrast, the NADP/NADPH
Nicotinamide adenine dinucleotide phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or TPN in older notation , is a coenzyme used in anabolic reactions, such as lipid and nucleic acid synthesis, which require NADPH as a reducing agent....
ratio is normally about 0.005, so NADPH is the dominant form of this coenzyme. These different ratios are key to the different metabolic roles of NADH and NADPH.
Biosynthesis
NAD is synthesized through two metabolic pathways. It is produced either in a de novoDe novo synthesis
De novo synthesis refers to the synthesis of complex molecules from simple molecules such as sugars or amino acids, as opposed to their being recycled after partial degradation. For example, nucleotides are not needed in the diet as they can be constructed from small precursor molecules such as...
pathway from amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
s or in salvage pathways by recycling preformed components such as nicotinamide
Nicotinamide
Nicotinamide, also known as niacinamide and nicotinic acid amide, is the amide of nicotinic acid . Nicotinamide is a water-soluble vitamin and is part of the vitamin B group...
back to NAD.
De novo production
Most organisms synthesize NAD from simple components. The specific set of reactions differs among organisms, but a common feature is the generation of quinolinic acidQuinolinic acid
Quinolinic acid is a dicarboxylic acid. It may be prepared by the oxidation of quinoline, either electrochemically, or with acidic hydrogen peroxide.Quinolinic acid is a downstream kynurenine pathway metabolite of tryptophan...
(QA) from an amino acid—either tryptophan
Tryptophan
Tryptophan is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. It is encoded in the standard genetic code as the codon UGG...
(Trp) in animals and some bacteria, or aspartic acid
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
in some bacteria and plants. The quinolinic acid is converted to nicotinic acid mononucleotide (NaMN) by transfer of a phosphoribose moiety. An adenylate moiety is then transferred to form nicotinic acid adenine dinucleotide (NaAD). Finally, the nicotinic acid moiety in NaAD is amidated
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
to a nicotinamide (Nam) moiety, forming nicotinamide adenine dinucleotide.
In a further step, some NAD is converted into NADP by NAD+ kinase
NAD+ kinase
NAD+ kinase is an enzyme that converts nicotinamide adenine dinucleotide into NADP+, through phosphorylating the NAD+ coenzyme. NADP+ is an essential coenzyme in metabolism and provides reducing power to biosynthetic processes such as fatty acid biosynthesis...
, which phosphorylates NAD. In most organisms, this enzyme uses ATP as the source of the phosphate group, although several bacteria such as Mycobacterium tuberculosis
Mycobacterium tuberculosis
Mycobacterium tuberculosis is a pathogenic bacterial species in the genus Mycobacterium and the causative agent of most cases of tuberculosis . First discovered in 1882 by Robert Koch, M...
and a hyperthermophilic archaeon Pyrococcus horikoshii
Pyrococcus
In taxonomy, Pyrococcus is a genus of the Thermococcaceae.- Description and significance :Pyrococcus has similar characteristics of other archaea such as Archaeoglobus, Thermoautotrophican, and Methanococcus in the respect that they are all thermophilic and anaerobic...
, use inorganic polyphosphate
Polyphosphate
Triphosphates are salts or esters of polymeric oxyanions formed from tetrahedral PO4 structural units linked together by sharing oxygen atoms. When two corners are shared the polyphosphate may have a linear chain structure or a cyclic ring structure. In biology the polyphosphate esters AMP, ADP...
as an alternative phosphoryl donor.
Salvage pathways
Besides assembling NAD de novo from simple amino acid precursors, cells also salvage preformed compounds containing nicotinamide. Although other precursors are known, the three natural compounds containing the nicotinamide ring and used in these salvage metabolic pathways are nicotinic acid (Na), nicotinamide (Nam) and nicotinamide ribosideRiboside
A riboside is any glycoside of ribose. Ribosides in the form of ribonucleosides and ribonucleotides play an important role in biochemistry....
(NR). These compounds can be taken up from the diet, where the mixture of nicotinic acid and nicotinamide are called vitamin B or niacin
Niacin
"Niacin" redirects here. For the neo-fusion band, see Niacin .Niacin is an organic compound with the formula and, depending on the definition used, one of the forty to eighty essential human nutrients.Niacin is one of five vitamins associated with a pandemic deficiency disease: niacin deficiency...
. However, these compounds are also produced within cells, when the nicotinamide moiety is released from NAD in ADP-ribose transfer reactions. Indeed, the enzymes involved in these salvage pathways appear to be concentrated in the cell nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
, which may compensate for the high level of reactions that consume NAD in this organelle
Organelle
In cell biology, an organelle is a specialized subunit within a cell that has a specific function, and is usually separately enclosed within its own lipid bilayer....
. Cells can also take up extracellular NAD from their surroundings.
Despite the presence of the de novo pathway, the salvage reactions are essential in humans; a lack of niacin in the diet causes the vitamin deficiency disease pellagra
Pellagra
Pellagra is a vitamin deficiency disease most commonly caused by a chronic lack of niacin in the diet. It can be caused by decreased intake of niacin or tryptophan, and possibly by excessive intake of leucine. It may also result from alterations in protein metabolism in disorders such as carcinoid...
. This high requirement for NAD results from the constant consumption of the coenzyme in reactions such as posttranslational modifications, since the cycling of NAD between oxidized and reduced forms in redox reactions does not change the overall levels of the coenzyme.
The salvage pathways used in microorganism
Microorganism
A microorganism or microbe is a microscopic organism that comprises either a single cell , cell clusters, or no cell at all...
s differ from those of mammal
Mammal
Mammals are members of a class of air-breathing vertebrate animals characterised by the possession of endothermy, hair, three middle ear bones, and mammary glands functional in mothers with young...
s. Some pathogens, such as the yeast Candida glabrata
Candida glabrata
Candida glabrata is a haploid yeast of the genus Candida, previously known as Torulopsis glabrata. This species of yeast is non-dimorphic and no mating activity has been observed. Until recently, C. glabrata was thought to be a primarily non-pathogenic organism. However, with the ever increasing...
and the bacterium Haemophilus influenzae
Haemophilus influenzae
Haemophilus influenzae, formerly called Pfeiffer's bacillus or Bacillus influenzae, Gram-negative, rod-shaped bacterium first described in 1892 by Richard Pfeiffer during an influenza pandemic. A member of the Pasteurellaceae family, it is generally aerobic, but can grow as a facultative anaerobe. H...
are NAD auxotrophs – they cannot synthesize NAD – but possess salvage pathways and thus are dependent on external sources of NAD or its precursors. Even more surprising is the intracellular pathogen
Pathogen
A pathogen gignomai "I give birth to") or infectious agent — colloquially, a germ — is a microbe or microorganism such as a virus, bacterium, prion, or fungus that causes disease in its animal or plant host...
Chlamydia trachomatis
Chlamydia trachomatis
Chlamydia trachomatis, an obligate intracellular human pathogen, is one of three bacterial species in the genus Chlamydia. C. trachomatis is a Gram-negative bacteria, therefore its cell wall components retain the counter-stain safranin and appear pink under a light microscope.The inclusion bodies...
, which lacks recognizable candidates for any genes involved in the biosynthesis or salvage of both NAD and NADP, and must acquire these coenzymes from its host
Host (biology)
In biology, a host is an organism that harbors a parasite, or a mutual or commensal symbiont, typically providing nourishment and shelter. In botany, a host plant is one that supplies food resources and substrate for certain insects or other fauna...
.
Functions
Nicotinamide adenine dinucleotide has several essential roles in metabolismMetabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
. It acts as a coenzyme in redox
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
reactions, as a donor of ADP-ribose moieties in ADP-ribosylation
ADP-ribosylation
ADP-ribosylation is the addition of one or more ADP-ribose moieties to a protein. These reactions are involved in cell signaling and the control of many cell processes, including DNA repair and apoptosis.-ADP-ribosylation enzymes:...
reactions, as a precursor of the second messenger
Second messenger system
Second messengers are molecules that relay signals from receptors on the cell surface to target molecules inside the cell, in the cytoplasm or nucleus. They relay the signals of hormones like epinephrine , growth factors, and others, and cause some kind of change in the activity of the cell...
molecule cyclic ADP-ribose
Cyclic ADP-ribose
Cyclic ADP Ribose, frequently abbreviated as cADPR, is a cyclic adenine nucleotide with two phosphate groups present on 5' OH of the adenosine , further connected to another ribose at the 5' position, which, in turn, closes the cycle by glycosidic bonding to the nitrogen 1 of the same adenine...
, as well as acting as a substrate for bacterial DNA ligase
DNA ligase
In molecular biology, DNA ligase is a specific type of enzyme, a ligase, that repairs single-stranded discontinuities in double stranded DNA molecules, in simple words strands that have double-strand break . Purified DNA ligase is used in gene cloning to join DNA molecules together...
s and a group of enzymes called sirtuin
Sirtuin
Sirtuin or Sir2 proteins are a class of proteins that possess either histone deacetylase or mono-ribosyltransferase activity. Sirtuins regulate important biological pathways in bacteria, archaea and eukaryotes...
s that use NAD to remove acetyl groups
Acetyl
In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl...
from proteins.
Oxidoreductase Binding
The main role of NAD in metabolism is the transfer of electrons from one molecule to another. Reactions of this type are catalyzed by a large group of enzymes called oxidoreductaseOxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule to another...
s. The correct names for these enzymes contain the names of both their substrates: for example NADH-ubiquinone oxidoreductase
NADH dehydrogenase
NADH dehydrogenase is an enzyme located in the inner mitochondrial membrane that catalyzes the transfer of electrons from NADH to coenzyme Q...
catalyzes the oxidation of NADH by coenzyme Q
Coenzyme Q
Coenzyme Q10, also known as ubiquinone, ubidecarenone, coenzyme Q, and abbreviated at times to CoQ10 , CoQ, Q10, or Q, is a 1,4-benzoquinone, where Q refers to the quinone chemical group, and 10 refers to the number of isoprenyl chemical subunits in its tail.This oil-soluble, vitamin-like substance...
. However, these enzymes are also referred to as dehydrogenases or reductases, with NADH-ubiquinone oxidoreductase commonly being called NADH dehydrogenase or sometimes coenzyme Q reductase.
When bound to a protein, NAD and NADH are usually held within a structural motif
Structural motif
In a chain-like biological molecule, such as a protein or nucleic acid, a structural motif is a supersecondary structure, which appears also in a variety of other molecules...
known as the Rossmann fold
Rossmann fold
The Rossmann fold is a protein structural motif found in proteins that bind nucleotides, especially the cofactor NAD. The structure with two repeats is composed of six parallel beta strands linked to two pairs of alpha helices in the topological order beta-alpha-beta-alpha-beta...
. The motif is named after Michael Rossmann who was the first scientist to notice how common this structure is within nucleotide-binding proteins. This fold contains three or more parallel beta strands
Beta sheet
The β sheet is the second form of regular secondary structure in proteins, only somewhat less common than the alpha helix. Beta sheets consist of beta strands connected laterally by at least two or three backbone hydrogen bonds, forming a generally twisted, pleated sheet...
linked by two alpha helices
Alpha helix
A common motif in the secondary structure of proteins, the alpha helix is a right-handed coiled or spiral conformation, in which every backbone N-H group donates a hydrogen bond to the backbone C=O group of the amino acid four residues earlier...
in the order beta-alpha-beta-alpha-beta. This forms a beta sheet flanked by a layer of alpha helices on each side. Because each Rossmann fold binds one nucleotide, binding domains for the dinucleotide NAD consist of two paired Rossmann folds, with each fold binding one nucleotide within the cofactor. However, this fold is not universal among NAD-dependent enzymes, since a class of bacterial enzymes involved in amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
metabolism have recently been discovered that bind the coenzyme, but lack this motif.
When bound in the active site of an oxidoreductase, the nicotinamide ring of the coenzyme is positioned so that it can accept a hydride from the other substrate. Since the C4 carbon that accepts the hydrogen is prochiral
Prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step.If two identical substituents are attached to a sp3-hybridized atom, the descriptors pro-R and pro-S are used to distinguish between the two...
, this can be exploited in enzyme kinetics
Enzyme kinetics
Enzyme kinetics is the study of the chemical reactions that are catalysed by enzymes. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction investigated...
to give information about the enzyme's mechanism. This is done by mixing an enzyme with a substrate that has deuterium
Deuterium
Deuterium, also called heavy hydrogen, is one of two stable isotopes of hydrogen. It has a natural abundance in Earth's oceans of about one atom in of hydrogen . Deuterium accounts for approximately 0.0156% of all naturally occurring hydrogen in Earth's oceans, while the most common isotope ...
atoms substituted for the hydrogens, so the enzyme will reduce NAD by transferring deuterium rather than hydrogen. In this case, an enzyme can produce one of two stereoisomer
Stereoisomerism
Stereoisomers are isomeric molecules that have the same molecular formula and sequence of bonded atoms , but that differ only in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections...
s of NADH. In some enzymes the hydrogen is transferred from above the plane of the nicotinamide ring; these are called class A oxidoreductases, whereas class B enzymes transfer the atom from below.
Despite the similarity in how proteins bind the two coenzymes, enzymes almost always show a high level of specificity for either NAD or NADP. This specificity reflects the distinct metabolic roles of the respective coenzymes, and is the result of distinct sets of amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
residues in the two types of coenzyme-binding pocket. For instance, in the active site of NADP-dependent enzymes, an ionic bond
Ionic bond
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. Pure ionic bonding cannot exist: all ionic compounds have some...
is formed between a basic amino acid side-chain and the acidic phosphate group of NADP. On the converse, in NAD-dependent enzymes the charge in this pocket is reversed, preventing NADP from binding. However, there are a few exceptions to this general rule, and enzymes such as aldose reductase
Aldose reductase
Aldose reductase is an NADPH-dependent oxidoreductase that catalyzes the reduction of a variety of aldehydes and carbonyls, including monosaccharides...
, glucose-6-phosphate dehydrogenase
Glucose-6-phosphate dehydrogenase
Glucose-6-phosphate dehydrogenase is a cytosolic enzyme in the pentose phosphate pathway , a metabolic pathway that supplies reducing energy to cells by maintaining the level of the co-enzyme nicotinamide adenine dinucleotide phosphate...
, and methylenetetrahydrofolate reductase
Methylenetetrahydrofolate reductase
Methylenetetrahydrofolate reductase is an enzyme that in humans is encoded by the MTHFR gene. Methylenetetrahydrofolate reductase catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a cosubstrate for homocysteine remethylation to methionine...
can use both coenzymes in some species.
Role in redox metabolism

The redox reactions catalyzed by oxidoreductases are vital in all parts of metabolism, but one particularly important area where these reactions occur is in the release of energy from nutrients. Here, reduced compounds such as glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
and fatty acids are oxidized, thereby releasing energy. This energy is transferred to NAD by reduction to NADH, as part of beta oxidation
Beta oxidation
Beta oxidation is the process by which fatty acids, in the form of Acyl-CoA molecules, are broken down in mitochondria and/or in peroxisomes to generate Acetyl-CoA, the entry molecule for the Citric Acid cycle....
, glycolysis
Glycolysis
Glycolysis is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+...
, and the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
. In eukaryote
Eukaryote
A eukaryote is an organism whose cells contain complex structures enclosed within membranes. Eukaryotes may more formally be referred to as the taxon Eukarya or Eukaryota. The defining membrane-bound structure that sets eukaryotic cells apart from prokaryotic cells is the nucleus, or nuclear...
s the electrons carried by the NADH that is produced in the cytoplasm
Cytoplasm
The cytoplasm is a small gel-like substance residing between the cell membrane holding all the cell's internal sub-structures , except for the nucleus. All the contents of the cells of prokaryote organisms are contained within the cytoplasm...
are transferred into the mitochondrion
Mitochondrion
In cell biology, a mitochondrion is a membrane-enclosed organelle found in most eukaryotic cells. These organelles range from 0.5 to 1.0 micrometers in diameter...
(to reduce mitochondrial NAD) by mitochondrial shuttle
Mitochondrial shuttle
The mitochondrial shuttles are systems used to transport reducing agents across the inner mitochondrial membrane. NADH cannot cross the membrane, but it can reduce another molecule that can cross the membrane, so that its electrons can reach the electron transport chain.The two main systems in...
s, such as the malate-aspartate shuttle
Malate-aspartate shuttle
The malate-aspartate shuttle is a biochemical system for translocating electrons produced during glycolysis across the semipermeable inner membrane of the mitochondrion for oxidative phosphorylation in eukaryotes. These electrons enter the electron transport chain of the mitochondria via reduction...
. The mitochondrial NADH is then oxidized in turn by the electron transport chain
Electron transport chain
An electron transport chain couples electron transfer between an electron donor and an electron acceptor with the transfer of H+ ions across a membrane. The resulting electrochemical proton gradient is used to generate chemical energy in the form of adenosine triphosphate...
, which pumps protons across a membrane and generates ATP through oxidative phosphorylation
Oxidative phosphorylation
Oxidative phosphorylation is a metabolic pathway that uses energy released by the oxidation of nutrients to produce adenosine triphosphate . Although the many forms of life on earth use a range of different nutrients, almost all aerobic organisms carry out oxidative phosphorylation to produce ATP,...
. These shuttle systems also have the same transport function in chloroplast
Chloroplast
Chloroplasts are organelles found in plant cells and other eukaryotic organisms that conduct photosynthesis. Chloroplasts capture light energy to conserve free energy in the form of ATP and reduce NADP to NADPH through a complex set of processes called photosynthesis.Chloroplasts are green...
s.
Since both the oxidized and reduced forms of nicotinamide adenine dinucleotide are used in these linked sets of reactions, the cell maintains significant concentrations of both NAD and NADH, with the high NAD/NADH ratio allowing this coenzyme to act as both an oxidizing and a reducing agent. In contrast, the main function of NADPH is as a reducing agent in anabolism
Anabolism
Anabolism is the set of metabolic pathways that construct molecules from smaller units. These reactions require energy. One way of categorizing metabolic processes, whether at the cellular, organ or organism level is as 'anabolic' or as 'catabolic', which is the opposite...
, with this coenzyme being involved in pathways such as fatty acid synthesis
Fatty acid synthesis
Fatty acid synthesis is the creation of fatty acids from acetyl-CoA and malonyl-CoA precursors through action of enzymes called fatty acid synthases...
and photosynthesis
Photosynthesis
Photosynthesis is a chemical process that converts carbon dioxide into organic compounds, especially sugars, using the energy from sunlight. Photosynthesis occurs in plants, algae, and many species of bacteria, but not in archaea. Photosynthetic organisms are called photoautotrophs, since they can...
. Since NADPH is needed to drive redox reactions as a strong reducing agent, the NADP/NADPH ratio is kept very low.
Although it is important in catabolism, NADH is also used in anabolic reactions, such as gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
. This need for NADH in anabolism poses a problem for prokaryotes growing on nutrients that release only a small amount of energy. For example, nitrifying
Nitrification
Nitrification is the biological oxidation of ammonia with oxygen into nitrite followed by the oxidation of these nitrites into nitrates. Degradation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle in soil...
bacteria such as Nitrobacter
Nitrobacter
Nitrobacter is genus of mostly rod-shaped, gram-negative, and chemoautotrophic bacteria.Nitrobacter plays an important role in the nitrogen cycle by oxidizing nitrite into nitrate in soil...
oxidize nitrite to nitrate, which releases sufficient energy to pump protons and generate ATP, but not enough to produce NADH directly. As NADH is still needed for anabolic reactions, these bacteria use a nitrite oxidoreductase
Nitrite oxidoreductase
Nitrite oxidoreductase is an enzyme involved in nitrification. It is the last step in the process of aerobic ammonia oxidation, which is carried out by two groups of nitrifying bacteria: ammonia oxidizers such as Nitrosospira, Nitrosomonas and Nitrosococcus convert ammonia to nitrite, while...
to produce enough proton-motive force
Chemiosmosis
Chemiosmosis is the movement of ions across a selectively permeable membrane, down their electrochemical gradient. More specifically, it relates to the generation of ATP by the movement of hydrogen ions across a membrane during cellular respiration....
to run part of the electron transport chain in reverse, generating NADH.
Non-redox roles
The coenzyme NAD is also consumed in ADP-ribose transfer reactions. For example, enzymes called ADP-ribosyltransferasesGlycosyltransferase
Glycosyltransferases are enzymes that act as a catalyst for the transfer of a monosaccharide unit from an activated nucleotide sugar to a glycosyl acceptor molecule, usually an alcohol....
add the ADP-ribose moiety of this molecule to proteins, in a posttranslational modification
Posttranslational modification
Posttranslational modification is the chemical modification of a protein after its translation. It is one of the later steps in protein biosynthesis, and thus gene expression, for many proteins....
called ADP-ribosylation
ADP-ribosylation
ADP-ribosylation is the addition of one or more ADP-ribose moieties to a protein. These reactions are involved in cell signaling and the control of many cell processes, including DNA repair and apoptosis.-ADP-ribosylation enzymes:...
. NAD may also be added onto cellular RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
as a base
Nucleobase
Nucleobases are a group of nitrogen-based molecules that are required to form nucleotides, the basic building blocks of DNA and RNA. Nucleobases provide the molecular structure necessary for the hydrogen bonding of complementary DNA and RNA strands, and are key components in the formation of stable...
modification. ADP-ribosylation involves either the addition of a single ADP-ribose moiety, in mono-ADP-ribosylation, or the transferral of ADP-ribose to proteins in long branched chains, which is called poly(ADP-ribosyl)ation. Mono-ADP-ribosylation was first identified as the mechanism of a group of bacterial toxin
Toxin
A toxin is a poisonous substance produced within living cells or organisms; man-made substances created by artificial processes are thus excluded...
s, notably cholera toxin
Cholera toxin
Cholera toxin is a protein complex secreted by the bacterium Vibrio cholerae. CTX is responsible for the massive, watery diarrhea characteristic of cholera infection.- Structure :...
, but it is also involved in normal cell signaling
Cell signaling
Cell signaling is part of a complex system of communication that governs basic cellular activities and coordinates cell actions. The ability of cells to perceive and correctly respond to their microenvironment is the basis of development, tissue repair, and immunity as well as normal tissue...
. Poly(ADP-ribosyl)ation is carried out by the poly(ADP-ribose) polymerase
Poly ADP ribose polymerase
Poly polymerase is a family of proteins involved in a number of cellular processes involving mainly DNA repair and programmed cell death.-Members of PARP family:The PARP family comprises 17 members...
s. The poly(ADP-ribose) structure is involved in the regulation of several cellular events and is most important in the cell nucleus
Cell nucleus
In cell biology, the nucleus is a membrane-enclosed organelle found in eukaryotic cells. It contains most of the cell's genetic material, organized as multiple long linear DNA molecules in complex with a large variety of proteins, such as histones, to form chromosomes. The genes within these...
, in processes such as DNA repair
DNA repair
DNA repair refers to a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as UV light and radiation can cause DNA damage, resulting in as many as 1...
and telomere
Telomere
A telomere is a region of repetitive DNA sequences at the end of a chromosome, which protects the end of the chromosome from deterioration or from fusion with neighboring chromosomes. Its name is derived from the Greek nouns telos "end" and merοs "part"...
maintenance. In addition to these functions within the cell, a group of extracellular
Extracellular
In cell biology, molecular biology and related fields, the word extracellular means "outside the cell". This space is usually taken to be outside the plasma membranes, and occupied by fluid...
ADP-ribosyltransferases has recently been discovered, but their functions remain obscure.
Another function of this coenzyme in cell signaling is as a precursor of cyclic ADP-ribose
Cyclic ADP-ribose
Cyclic ADP Ribose, frequently abbreviated as cADPR, is a cyclic adenine nucleotide with two phosphate groups present on 5' OH of the adenosine , further connected to another ribose at the 5' position, which, in turn, closes the cycle by glycosidic bonding to the nitrogen 1 of the same adenine...
, which is produced from NAD by ADP-ribosyl cyclases, as part of a second messenger system
Second messenger system
Second messengers are molecules that relay signals from receptors on the cell surface to target molecules inside the cell, in the cytoplasm or nucleus. They relay the signals of hormones like epinephrine , growth factors, and others, and cause some kind of change in the activity of the cell...
. This molecule acts in calcium signaling
Calcium signaling
Calcium is a common signaling mechanism, as once it enters the cytoplasm it exerts allosteric regulatory effects on many enzymes and proteins...
by releasing calcium from intracellular stores. It does this by binding to and opening a class of calcium channels called ryanodine receptor
Ryanodine receptor
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons...
s, which are located in the membranes of organelle
Organelle
In cell biology, an organelle is a specialized subunit within a cell that has a specific function, and is usually separately enclosed within its own lipid bilayer....
s, such as the endoplasmic reticulum
Endoplasmic reticulum
The endoplasmic reticulum is an organelle of cells in eukaryotic organisms that forms an interconnected network of tubules, vesicles, and cisternae...
.
NAD is also consumed by sirtuin
Sirtuin
Sirtuin or Sir2 proteins are a class of proteins that possess either histone deacetylase or mono-ribosyltransferase activity. Sirtuins regulate important biological pathways in bacteria, archaea and eukaryotes...
s, which are NAD-dependent deacetylases
Histone deacetylase
Histone deacetylases are a class of enzymes that remove acetyl groups from an ε-N-acetyl lysine amino acid on a histone. This is important because DNA is wrapped around histones, and DNA expression is regulated by acetylation and de-acetylation. Its action is opposite to that of histone...
, such as Sir2
Sir2
Sir2 was the first gene of the sirtuin genes to be found. It was found in budding yeast, and, since then, members of this highly conserved family have been found in nearly all organisms studied...
. These enzymes act by transferring an acetyl
Acetyl
In organic chemistry, acetyl is a functional group, the acyl with chemical formula COCH3. It is sometimes represented by the symbol Ac . The acetyl group contains a methyl group single-bonded to a carbonyl...
group from their substrate protein to the ADP-ribose moiety of NAD; this cleaves the coenzyme and releases nicotinamide and O-acetyl-ADP-ribose. The sirtuins mainly seem to be involved in regulating transcription
Transcription (genetics)
Transcription is the process of creating a complementary RNA copy of a sequence of DNA. Both RNA and DNA are nucleic acids, which use base pairs of nucleotides as a complementary language that can be converted back and forth from DNA to RNA by the action of the correct enzymes...
through deacetylating histones and altering nucleosome
Nucleosome
Nucleosomes are the basic unit of DNA packaging in eukaryotes, consisting of a segment of DNA wound around a histone protein core. This structure is often compared to thread wrapped around a spool....
structure. However, non-histone proteins can be deacetylated by sirtuins as well. These activities of sirtuins are particularly interesting because of their importance in the regulation of aging.
Other NAD-dependent enzymes include bacterial DNA ligase
DNA ligase
In molecular biology, DNA ligase is a specific type of enzyme, a ligase, that repairs single-stranded discontinuities in double stranded DNA molecules, in simple words strands that have double-strand break . Purified DNA ligase is used in gene cloning to join DNA molecules together...
s, which join two DNA ends by using NAD as a substrate to donate an adenosine monophosphate
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
(AMP) moiety to the 5' phosphate of one DNA end. This intermediate is then attacked by the 3' hydroxyl group of the other DNA end, forming a new phosphodiester bond
Phosphodiester bond
A phosphodiester bond is a group of strong covalent bonds between a phosphate group and two 5-carbon ring carbohydrates over two ester bonds. Phosphodiester bonds are central to all known life, as they make up the backbone of each helical strand of DNA...
. This contrasts with eukaryotic DNA ligases, which use ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
to form the DNA-AMP intermediate.
Pharmacology and medical uses
The enzymes that make and use NAD and NADH are important in both current pharmacologyPharmacology
Pharmacology is the branch of medicine and biology concerned with the study of drug action. More specifically, it is the study of the interactions that occur between a living organism and chemicals that affect normal or abnormal biochemical function...
and the research into future treatments for disease. Drug design
Drug design
Drug design, also sometimes referred to as rational drug design or structure-based drug design, is the inventive process of finding new medications based on the knowledge of the biological target...
and drug development exploits NAD in three ways: as a direct target of drugs, by designing enzyme inhibitor
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
s or activators based on its structure that change the activity of NAD-dependent enzymes, and by trying to inhibit NAD biosynthesis.
The coenzyme NAD is not itself currently used as a treatment for any disease. However, it is potentially useful in the therapy of neurodegenerative diseases such as Alzheimer's
Alzheimer's disease
Alzheimer's disease also known in medical literature as Alzheimer disease is the most common form of dementia. There is no cure for the disease, which worsens as it progresses, and eventually leads to death...
and Parkinson disease. Evidence on the use of NAD in neurodegeneration
NAD+ in neurodegeneration
Considering the importance of NAD+ in energy metabolism, DNA repair and transcriptional regulation, maintaining intracellular NAD+ reserves emerges as a major therapeutic target for the treatment of several age-related degenerative diseases, including Alzheimer's disease...
is mixed; studies in mice
Mouse
A mouse is a small mammal belonging to the order of rodents. The best known mouse species is the common house mouse . It is also a popular pet. In some places, certain kinds of field mice are also common. This rodent is eaten by large birds such as hawks and eagles...
are promising, whereas a placebo-controlled clinical trial failed to show any effect. NAD is also a direct target of the drug isoniazid
Isoniazid
Isoniazid , also known as isonicotinylhydrazine , is an organic compound that is the first-line antituberculosis medication in prevention and treatment. It was first discovered in 1912, and later in 1951 it was found to be effective against tuberculosis by inhibiting its mycolic acid...
, which is used in the treatment of tuberculosis
Tuberculosis
Tuberculosis, MTB, or TB is a common, and in many cases lethal, infectious disease caused by various strains of mycobacteria, usually Mycobacterium tuberculosis. Tuberculosis usually attacks the lungs but can also affect other parts of the body...
, an infection caused by Mycobacterium tuberculosis
Mycobacterium tuberculosis
Mycobacterium tuberculosis is a pathogenic bacterial species in the genus Mycobacterium and the causative agent of most cases of tuberculosis . First discovered in 1882 by Robert Koch, M...
. Isoniazid is a prodrug
Prodrug
A prodrug is a pharmacological substance administered in an inactive form. Once administered, the prodrug is metabolised in vivo into an active metabolite, a process termed bioactivation. The rationale behind the use of a prodrug is generally for absorption, distribution, metabolism, and...
and once it has entered the bacteria, it is activated by a peroxidase
Peroxidase
Peroxidases are a large family of enzymes that typically catalyze a reaction of the form:For many of these enzymes the optimal substrate is hydrogen peroxide, but others are more active with organic hydroperoxides such as lipid peroxides...
, which oxidizes the compound into a free radical form. This radical then reacts with NADH, to produce adducts that are very potent inhibitors of the enzymes enoyl-acyl carrier protein reductase
Enoyl-acyl carrier protein reductase
Enoyl-acyl carrier protein reductase , is a key enzyme of the type II fatty acid synthesis system. ENR is an attractive target for narrow-spectrum antibacterial drug discovery because of its essential role in metabolism and its sequence conservation across many bacterial species...
, and dihydrofolate reductase
Dihydrofolate reductase
- Function :Dihydrofolate reductase converts dihydrofolate into tetrahydrofolate, a methyl group shuttle required for the de novo synthesis of purines, thymidylic acid, and certain amino acids...
.
Since a large number of oxidoreductases use NAD and NADH as substrates, and bind them using a highly conserved structural motif, the idea that inhibitors based on NAD could be specific to one enzyme is surprising. However, this can be possible: for example, inhibitors based on the compounds mycophenolic acid
Mycophenolic acid
Mycophenolic acid INN or mycophenolate is an immunosuppressant drug used to prevent rejection in organ transplantation. It inhibits an enzyme needed for the growth of T cells and B cells. It was initially marketed as the prodrug mycophenolate mofetil to improve oral bioavailability. More...
and tiazofurin
Tiazofurin
Tiazofurin is an inhibitor of IMP dehydrogenase. Tiazofurin and its analogues are under investigation for potential use in the treatment of cancer....
inhibit IMP dehydrogenase
IMP dehydrogenase
IMP dehydrogenase is an enzyme that converts inosine monophosphate to xanthosine monophosphate:The structure of this enzyme is composed of a TIM barrel domain with two CBS domains inserted within a loop.It is inhibited by Mycophenolic acid.- Examples :...
at the NAD binding site. Because of the importance of this enzyme in purine metabolism
Purine metabolism
-Biosynthesis:Purines are biologically synthesized as nucleotides and in particular as ribotides, i.e. bases attached to ribose 5-phosphate. A key regulatory step is the production of 5-phospho-α-D-ribosyl 1-pyrophosphate by PRPP synthetase, which is activated by inorganic phosphate and...
, these compounds may be useful as anti-cancer, anti-viral, or immunosuppressive drug
Immunosuppressive drug
Immunosuppressive drugs or immunosuppressive agents are drugs that inhibit or prevent activity of the immune system. They are used in immunosuppressive therapy to:...
s. Other drugs are not enzyme inhibitors, but instead activate enzymes involved in NAD metabolism. Sirtuin
Sirtuin
Sirtuin or Sir2 proteins are a class of proteins that possess either histone deacetylase or mono-ribosyltransferase activity. Sirtuins regulate important biological pathways in bacteria, archaea and eukaryotes...
s are a particularly interesting target for such drugs, since activation of these NAD-dependent deacetylases extends lifespan. Compounds such as resveratrol
Resveratrol
Resveratrol is a stilbenoid, a type of natural phenol, and a phytoalexin produced naturally by several plants when under attack by pathogens such as bacteria or fungi....
increase the activity of these enzymes, which may be important in their ability to delay aging in both vertebrate, and invertebrate model organism
Model organism
A model organism is a non-human species that is extensively studied to understand particular biological phenomena, with the expectation that discoveries made in the organism model will provide insight into the workings of other organisms. Model organisms are in vivo models and are widely used to...
s.
Because of the differences in the metabolic pathway
Metabolic pathway
In biochemistry, metabolic pathways are series of chemical reactions occurring within a cell. In each pathway, a principal chemical is modified by a series of chemical reactions. Enzymes catalyze these reactions, and often require dietary minerals, vitamins, and other cofactors in order to function...
s of NAD biosynthesis between organisms, such as between bacteria and humans, this area of metabolism is a promising area for the development of new antibiotic
Antibiotic
An antibacterial is a compound or substance that kills or slows down the growth of bacteria.The term is often used synonymously with the term antibiotic; today, however, with increased knowledge of the causative agents of various infectious diseases, antibiotic has come to denote a broader range of...
s. For example, the enzyme nicotinamidase
Nicotinamidase
In enzymology, a nicotinamidase is an enzyme that catalyzes the chemical reactionThus, the two substrates of this enzyme are nicotinamide and H2O, whereas its two products are nicotinate and NH3....
, which converts nicotinamide to nicotinic acid, is a target for drug design, as this enzyme is absent in humans but present in yeast and bacteria.
History
The coenzyme NAD was first discovered by the BritishUnited Kingdom
The United Kingdom of Great Britain and Northern IrelandIn the United Kingdom and Dependencies, other languages have been officially recognised as legitimate autochthonous languages under the European Charter for Regional or Minority Languages...
biochemists Arthur Harden
Arthur Harden
Sir Arthur Harden FRS was an English biochemist. He shared the Nobel Prize in Chemistry in 1929 with Hans Karl August Simon von Euler-Chelpin for their investigations into the fermentation of sugar and fermentative enzymes....
and William Youndin in 1906. They noticed that adding boiled and filtered yeast
Yeast
Yeasts are eukaryotic micro-organisms classified in the kingdom Fungi, with 1,500 species currently described estimated to be only 1% of all fungal species. Most reproduce asexually by mitosis, and many do so by an asymmetric division process called budding...
extract greatly accelerated alcoholic fermentation in unboiled yeast extracts. They called the unidentified factor responsible for this effect a coferment. Through a long and difficult purification from yeast extracts, this heat-stable factor was identified as a nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
sugar phosphate by Hans von Euler-Chelpin
Hans von Euler-Chelpin
Hans Karl August Simon von Euler-Chelpin was a German-born Swedish biochemist. He won the Nobel Prize in Chemistry in 1929 with Arthur Harden for their investigations on the fermentation of sugar and fermentative enzymes.He was professor of general and organic chemistry at Stockholm University...
. In 1936, the German
Germany
Germany , officially the Federal Republic of Germany , is a federal parliamentary republic in Europe. The country consists of 16 states while the capital and largest city is Berlin. Germany covers an area of 357,021 km2 and has a largely temperate seasonal climate...
scientist Otto Heinrich Warburg
Otto Heinrich Warburg
Otto Heinrich Warburg , son of physicist Emil Warburg, was a German physiologist, medical doctor and Nobel laureate. He served as an officer in the elite Uhlan during the First World War and won the Iron Cross for bravery. Warburg was one of the twentieth century's leading biochemists...
showed the function of the nucleotide coenzyme in hydride transfer and identified the nicotinamide portion as the site of redox reactions.
A source of nicotinamide was identified in 1938, when Conrad Elvehjem
Conrad Elvehjem
Conrad A. Elvehjem, , was internationally known as a biochemist in nutrition. In 1937 he identified a molecule found in fresh meat and yeast as a new vitamin, nicotinic acid, now called niacin...
purified niacin
Niacin
"Niacin" redirects here. For the neo-fusion band, see Niacin .Niacin is an organic compound with the formula and, depending on the definition used, one of the forty to eighty essential human nutrients.Niacin is one of five vitamins associated with a pandemic deficiency disease: niacin deficiency...
from liver and showed this vitamin contained nicotinic acid and nicotinamide. Then, in 1939, he provided the first strong evidence that niacin was used to synthesize NAD. In the early 1940s, Arthur Kornberg
Arthur Kornberg
Arthur Kornberg was an American biochemist who won the Nobel Prize in Physiology or Medicine 1959 for his discovery of "the mechanisms in the biological synthesis of deoxyribonucleic acid " together with Dr. Severo Ochoa of New York University...
made another important contribution towards understanding NAD metabolism, by being the first to detect an enzyme in the biosynthetic pathway. Subsequently, in 1949, the American
United States
The United States of America is a federal constitutional republic comprising fifty states and a federal district...
biochemists Morris Friedkin and Albert L. Lehninger
Albert L. Lehninger
Albert Lester Lehninger was an American biochemist in the field of bioenergetics. He made fundamental contributions to the current understanding of metabolism at a molecular level. In 1948, he discovered, with Eugene P...
proved that NADH linked metabolic pathways such as the citric acid cycle with the synthesis of ATP in oxidative phosphorylation. Finally, in 1959, Jack Preiss and Philip Handler discovered the intermediates and enzymes involved in the biosynthesis of NAD; consequently, de novo synthesis is often called the Preiss-Handler pathway in their honor.
The non-redox roles of NAD(P) are a recent discovery. The first of these functions to be identified was the use of NAD as the ADP-ribose donor in ADP-ribosylation reactions, observed in the early 1960s. Later studies in the 1980s and 1990s revealed the activities of NAD and NADP metabolites in cell signaling – such as the action of cyclic ADP-ribose
Cyclic ADP-ribose
Cyclic ADP Ribose, frequently abbreviated as cADPR, is a cyclic adenine nucleotide with two phosphate groups present on 5' OH of the adenosine , further connected to another ribose at the 5' position, which, in turn, closes the cycle by glycosidic bonding to the nitrogen 1 of the same adenine...
, which was discovered in 1987. The metabolism of NAD has remained an area of intense research into the 21st century, with interest being heightened after the discovery of the NAD-dependent protein deacetylases called sirtuin
Sirtuin
Sirtuin or Sir2 proteins are a class of proteins that possess either histone deacetylase or mono-ribosyltransferase activity. Sirtuins regulate important biological pathways in bacteria, archaea and eukaryotes...
s in 2000, by Shinichiro Imai and coworkers at the Massachusetts Institute of Technology
Massachusetts Institute of Technology
The Massachusetts Institute of Technology is a private research university located in Cambridge, Massachusetts. MIT has five schools and one college, containing a total of 32 academic departments, with a strong emphasis on scientific and technological education and research.Founded in 1861 in...
.
Further reading
FunctionHistory, A history of early enzymology., A textbook from the 19th century.
External links
- NAD Animation (Flash Required)
- β-Nicotinamide adenine dinucleotide (NAD, oxidized) and NADH (reduced) Chemical data sheet from Sigma-AldrichSigma-AldrichSigma-Aldrich Corporation , is a life science and high technology company with over 7,600 employees and operations in 40 countries. Its chemical and biochemical products and kits are used in scientific research, biotechnology, pharmaceutical development, the diagnosis of disease, and as key...
- NAD, NADH and NAD synthesis pathway at the MetaCycMetaCycThe MetaCyc database contains extensive information on metabolic pathways and enzymes from many organisms. MetaCyc stores experimentally determined metabolic pathways....
database - List of oxidoreductases at the SWISS-PROT database

