Nitrification
Encyclopedia
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
of ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
with oxygen into nitrite
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
followed by the oxidation of these nitrites into nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
s. Degradation of ammonia to nitrite is usually the rate limiting step of nitrification. Nitrification is an important step in the nitrogen cycle
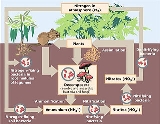
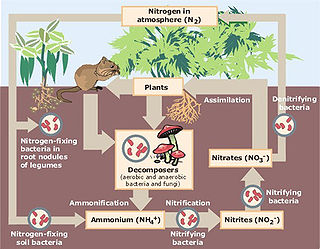
Nitrogen cycle
The nitrogen cycle is the process by which nitrogen is converted between its various chemical forms. This transformation can be carried out by both biological and non-biological processes. Important processes in the nitrogen cycle include fixation, mineralization, nitrification, and denitrification...
in soil
Soil
Soil is a natural body consisting of layers of mineral constituents of variable thicknesses, which differ from the parent materials in their morphological, physical, chemical, and mineralogical characteristics...
. This process was discovered by the Russia
Russia
Russia or , officially known as both Russia and the Russian Federation , is a country in northern Eurasia. It is a federal semi-presidential republic, comprising 83 federal subjects...
n microbiologist
Microbiologist
A microbiologist is a scientist who works in the field of microbiology. Microbiologists study organisms called microbes. Microbes can take the form of bacteria, viruses, fungi, and protists...
, Sergei Winogradsky
Sergei Winogradsky
Sergei Nikolaievich Winogradsky was a Ukrainian-Russian microbiologist, ecologist and soil scientist who pioneered the cycle of life concept. He discovered the first known form of lithotrophy during his research with Beggiatoa in 1887...
.
Microbiology and ecology
The oxidation of ammonia into nitrite is performed by two groups of organisms, ammonia-oxidizing bacteriaBacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
(AOB) and ammonia-oxidizing archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
(AOA).. AOB can be found among the betaproteobacteria
Betaproteobacteria
Betaproteobacteria is a class of Proteobacteria. Betaproteobacteria are, like all Proteobacteria, gram-negative.The Betaproteobacteria consist of several groups of aerobic or facultative bacteria which are often highly versatile in their degradation capacities, but also contain chemolithotrophic...
and gammaproteobacteria
Gammaproteobacteria
Gammaproteobacteria is a class of several medically, ecologically and scientifically important groups of bacteria, such as the Enterobacteriaceae , Vibrionaceae and Pseudomonadaceae. An exceeding number of important pathogens belongs to this class, e.g...
. Currently, only one AOA, Nitrosopumilus maritimus, has been isolated and described. In soils the most studied AOB belong to the genera Nitrosomonas
Nitrosomonas
Nitrosomonas is a genus comprising rod shaped chemoautotrophic bacteria.This rare bacteria oxidizes ammonia into nitrite as a metabolic process. Nitrosomonas are useful in treatment of industrial and sewage waste and in the process of bioremediation. They are important in the nitrogen cycle by...
and Nitrosococcus. Although in soils ammonia oxidation occurs by both AOB and AOA, AOA dominate in both soils and marine environments, suggesting that Crenarchaeota
Crenarchaeota
In taxonomy, the Crenarchaeota has been classified as either a phylum of the Archaea kingdom or a kingdom of its own...
may be greater contributors to ammonia oxidation in these environments.
The second step (oxidation of nitrite into nitrate) is done (mainly) by bacteria of the genus Nitrobacter
Nitrobacter
Nitrobacter is genus of mostly rod-shaped, gram-negative, and chemoautotrophic bacteria.Nitrobacter plays an important role in the nitrogen cycle by oxidizing nitrite into nitrate in soil...
. Both steps are producing energy to be coupled to ATP synthesis. Nitrifying organisms are chemoautotroph
Chemotroph
Chemotrophs are organisms that obtain energy by the oxidation of electron donors in their environments. These molecules can be organic or inorganic . The chemotroph designation is in contrast to phototrophs, which utilize solar energy...
s, and use carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
as their carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
source for growth. Some AOB possess the enzyme, urease, which catalyzes the conversion of the urea molecule to two ammonia molecules and one carbon dioxide molecule. Nitrosomonas europaea, as well as populations of soil-dwelling AOB, have been shown to assimilate the carbon dioxide released by the reaction to make biomass via the Calvin Cycle
Calvin cycle
The Calvin cycle or Calvin–Benson-Bassham cycle or reductive pentose phosphate cycle or C3 cycle or CBB cycle is a series of biochemical redox reactions that take place in the stroma of chloroplasts in photosynthetic organisms...
, and harvest energy by oxidizing ammonia (the other product of urease) to nitrite. This feature may explain enhanced growth of AOB in the presence of urea in acidic environments.
Nitrification also plays an important role in the removal of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
from municipal wastewater
Wastewater
Wastewater is any water that has been adversely affected in quality by anthropogenic influence. It comprises liquid waste discharged by domestic residences, commercial properties, industry, and/or agriculture and can encompass a wide range of potential contaminants and concentrations...
. The conventional removal is nitrification, followed by denitrification
Denitrification
Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen through a series of intermediate gaseous nitrogen oxide products....
. The cost of this process resides mainly in aeration
Aeration
Aeration is the process by which air is circulated through, mixed with or dissolved in a liquid or substance.-Aeration of liquids:-Methods:Aeration of liquids is achieved by:...
(bringing oxygen in the reactor) and the addition of an external carbon source (e.g., methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
) for the denitrification.
Nitrification can also occur in drinking water. In distribution systems where chloramines are used as the secondary disinfectant, the presence of free ammonia can act as a substrate for ammonia-oxidizing microorganisms. The associated reactions can lead to the depletion of the disinfectant residual in the system.
In most environments, both organisms are ford together, yielding nitrate as the final product. However, it is possible to design systems in which nitrite is formed (the Sharon process).
Together with ammonification, nitrification forms a mineralization
Mineralization (soil)
Mineralization in soil science, is when the chemical compounds in organic matter decompose or are oxidized into plant-accessible forms,. Mineralization is the opposite of immobilization....
process that refers to the complete decomposition of organic material, with the release of available nitrogen compounds. This replenishes the nitrogen cycle
Nitrogen cycle
The nitrogen cycle is the process by which nitrogen is converted between its various chemical forms. This transformation can be carried out by both biological and non-biological processes. Important processes in the nitrogen cycle include fixation, mineralization, nitrification, and denitrification...
.
Chemistry
Nitrification is a process of nitrogen compound oxidationRedox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
(effectively, loss of electrons from the nitrogen atom to the oxygen atoms):
- NH3 + 1.5 O2 + Nitrosomonas → NO2- + H2O + H+
- NO2- + 0.5 O2 + Nitrobacter → NO3-
- NH3 + O2 → NO2− + 3H+ + 2e−
- NO2− + H2O → NO3− + 2H+ + 2e−
Nitrification in the marine environment
In the marine environment, nitrogen is often the limiting nutrient, so the nitrogen cycleNitrogen cycle
The nitrogen cycle is the process by which nitrogen is converted between its various chemical forms. This transformation can be carried out by both biological and non-biological processes. Important processes in the nitrogen cycle include fixation, mineralization, nitrification, and denitrification...
in the ocean is of particular interest. The nitrification step of the cycle is of particular interest in the ocean because it creates nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
, the primary form of nitrogen responsible for “new” production
F-ratio
In oceanic biogeochemistry, the f-ratio is the fraction of total primary production fuelled by nitrate . This fraction is significant because it is assumed to be directly related to the sinking flux of organic marine snow from the surface ocean by the biological pump...
. Furthermore, as the ocean becomes enriched in anthropogenic
Anthropogenic
Human impact on the environment or anthropogenic impact on the environment includes impacts on biophysical environments, biodiversity and other resources. The term anthropogenic designates an effect or object resulting from human activity. The term was first used in the technical sense by Russian...
CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, the resulting decrease in pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
could lead to decreasing rates of nitrification. Nitrification could potentially become a “bottleneck” in the nitrogen cycle.
Nitrification, as stated above, is formally a two-step process; in the first step ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
is oxidized to nitrite
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
, and in the second step nitrite is oxidized to nitrate. Different microbes are responsible for each step in the marine environment. Several groups of ammonia oxidizing bacteria (AOB) are known in the marine environment, including Nitrosomonas
Nitrosomonas
Nitrosomonas is a genus comprising rod shaped chemoautotrophic bacteria.This rare bacteria oxidizes ammonia into nitrite as a metabolic process. Nitrosomonas are useful in treatment of industrial and sewage waste and in the process of bioremediation. They are important in the nitrogen cycle by...
, Nitrospira, and Nitrosococcus. All contain the functional gene ammonia monooxygenase (AMO) which, as its name implies, is responsible for the oxidation of ammonia. More recent metagenomic studies have revealed that some Crenarchaeote
Crenarchaeota
In taxonomy, the Crenarchaeota has been classified as either a phylum of the Archaea kingdom or a kingdom of its own...
Archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
possess AMO. Crenarchaeote are abundant in the ocean and some species have a 200 times greater affinity for ammonia than AOB, leading researchers to challenge the previous belief that AOB are primarily responsible for nitrification in the ocean. Furthermore, though nitrification is classically thought to be vertically separated from primary production
Primary production
400px|thumb|Global oceanic and terrestrial photoautotroph abundance, from September [[1997]] to August 2000. As an estimate of autotroph biomass, it is only a rough indicator of primary production potential, and not an actual estimate of it...
because the oxidation of nitrogen by bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
is inhibited by light, nitrification by AOA does not appear to be light inhibited, meaning that nitrification is occurring throughout the water column, challenging the classical definitions of “new” and “recycled” production
F-ratio
In oceanic biogeochemistry, the f-ratio is the fraction of total primary production fuelled by nitrate . This fraction is significant because it is assumed to be directly related to the sinking flux of organic marine snow from the surface ocean by the biological pump...
.
In the second step, nitrite is oxidized to nitrate. In the ocean, this step is not as well understood as the first, but the bacteria Nitrospina and Nitrobacter
Nitrobacter
Nitrobacter is genus of mostly rod-shaped, gram-negative, and chemoautotrophic bacteria.Nitrobacter plays an important role in the nitrogen cycle by oxidizing nitrite into nitrate in soil...
are known to carry out this step in the ocean.
--Brendmack (talk) 04:57, 27 October 2011 (UTC)
See also
- f-ratioF-ratioIn oceanic biogeochemistry, the f-ratio is the fraction of total primary production fuelled by nitrate . This fraction is significant because it is assumed to be directly related to the sinking flux of organic marine snow from the surface ocean by the biological pump...
- Haber processHaber processThe Haber process, also called the Haber–Bosch process, is the nitrogen fixation reaction of nitrogen gas and hydrogen gas, over an enriched iron or ruthenium catalyst, which is used to industrially produce ammonia....
- Nitrogen fixationNitrogen fixationNitrogen fixation is the natural process, either biological or abiotic, by which nitrogen in the atmosphere is converted into ammonia . This process is essential for life because fixed nitrogen is required to biosynthesize the basic building blocks of life, e.g., nucleotides for DNA and RNA and...
- Simultaneous nitrification-denitrificationSimultaneous nitrification-denitrificationSimultaneous nitrification-denitrification is a wastewater treatment process. Microbial simultaneous nitrification-denitrification is the conversion of the ammonium ion to nitrogen gas in a single bioreactor...
External links
- Nitrification at the heart of filtration at fishdoc.co.uk
- Nitrification at University of Aberdeen · King's College
- Nitrification Basics for Aerated Lagoon Operators at lagoonsonline.com

