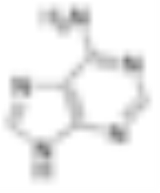
Nucleoside
Encyclopedia
| Nitrogenous base | Nucleoside | Deoxynucleoside |
|---|---|---|
 Adenine Adenine Adenine is a nucleobase with a variety of roles in biochemistry including cellular respiration, in the form of both the energy-rich adenosine triphosphate and the cofactors nicotinamide adenine dinucleotide and flavin adenine dinucleotide , and protein synthesis, as a chemical component of DNA... |
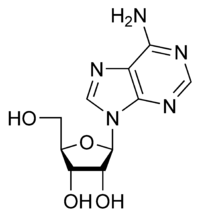 Adenosine A |
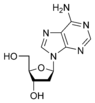 Deoxyadenosine Deoxyadenosine Deoxyadenosine is a deoxyribonucleoside. It is a derivative of the nucleoside adenosine, differing from the latter by the replacement of a hydroxyl group by hydrogen at the 2' position of its ribose sugar moiety. Deoxyadenosine is the DNA nucleoside A, which pairs with deoxythymidine in... dA |
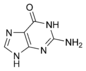 Guanine Guanine Guanine is one of the four main nucleobases found in the nucleic acids DNA and RNA, the others being adenine, cytosine, and thymine . In DNA, guanine is paired with cytosine. With the formula C5H5N5O, guanine is a derivative of purine, consisting of a fused pyrimidine-imidazole ring system with... |
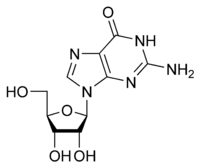 Guanosine Guanosine Guanosine is a purine nucleoside comprising guanine attached to a ribose ring via a β-N9-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate , cyclic guanosine monophosphate , guanosine diphosphate , and guanosine triphosphate... G |
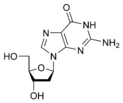 Deoxyguanosine Deoxyguanosine Deoxyguanosine is composed of the purine nucleoside guanine linked by its N9 nitrogen to the C1 carbon of deoxyribose. It is similar to guanosine, but with one hydroxyl group removed from the 2' position of the ribose sugar . If a phosphate group is attached at the 5' position, it becomes... dG |
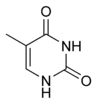 Thymine Thymine Thymine is one of the four nucleobases in the nucleic acid of DNA that are represented by the letters G–C–A–T. The others are adenine, guanine, and cytosine. Thymine is also known as 5-methyluracil, a pyrimidine nucleobase. As the name suggests, thymine may be derived by methylation of uracil at... |
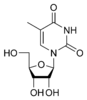 5-Methyluridine 5-Methyluridine The chemical compound 5-methyluridine, also called ribothymidine, is a pyrimidine nucleoside. It is the ribonucleoside counterpart to the deoxyribonucleoside thymidine, which lacks a hydroxyl group at the 2' position... m5U |
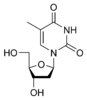 Thymidine Thymidine Thymidine is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine in double-stranded DNA... dT |
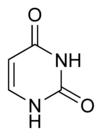 Uracil Uracil Uracil is one of the four nucleobases in the nucleic acid of RNA that are represented by the letters A, G, C and U. The others are adenine, cytosine, and guanine. In RNA, uracil binds to adenine via two hydrogen bonds. In DNA, the uracil nucleobase is replaced by thymine.Uracil is a common and... |
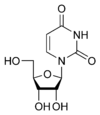 Uridine Uridine Uridine is a molecule that is formed when uracil is attached to a ribose ring via a β-N1-glycosidic bond.If uracil is attached to a deoxyribose ring, it is known as a deoxyuridine.... U |
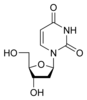 Deoxyuridine Deoxyuridine Deoxyuridine is a compound and a nucleoside. It is similar in chemical structure to uridine, but without the 2'-hydroxyl group.Idoxuridine and Trifluridine are variants of deoxyuridine used as antiviral drugs... dU |
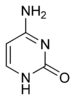 Cytosine Cytosine Cytosine is one of the four main bases found in DNA and RNA, along with adenine, guanine, and thymine . It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached . The nucleoside of cytosine is cytidine... |
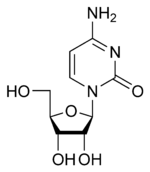 Cytidine Cytidine Cytidine is a nucleoside molecule that is formed when cytosine is attached to a ribose ring via a β-N1-glycosidic bond... C |
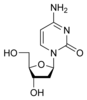 Deoxycytidine Deoxycytidine Deoxycytidine is a deoxyribonucleoside. It is like cytidine, but with one oxygen atom removed.... dC |
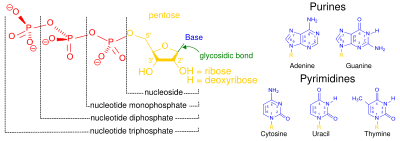
Nucleosides are glycosylamine
Glycosylamine
Glycosylamine is a class of biochemical compounds consisting of an amine with a β-N-glycosidic bond to a carbohydrate, thus forming a cyclic hemiaminal ether bond .Examples include nucleosides such as adenosine....
s consisting of a nucleobase
Nucleobase
Nucleobases are a group of nitrogen-based molecules that are required to form nucleotides, the basic building blocks of DNA and RNA. Nucleobases provide the molecular structure necessary for the hydrogen bonding of complementary DNA and RNA strands, and are key components in the formation of stable...
(often referred to as simply base) bound to a ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
or deoxyribose
Deoxyribose
Deoxyribose, more, precisely 2-deoxyribose, is a monosaccharide with idealized formula H---3-H. Its name indicates that it is a deoxy sugar, meaning that it is derived from the sugar ribose by loss of an oxygen atom...
sugar
Sugar
Sugar is a class of edible crystalline carbohydrates, mainly sucrose, lactose, and fructose, characterized by a sweet flavor.Sucrose in its refined form primarily comes from sugar cane and sugar beet...
via a beta
Anomer
In carbohydrate chemistry, an anomer is a special type of epimer. It is one of two stereoisomers of a cyclic saccharide that differs only in its configuration at the hemiacetal or hemiketal carbon, also called the anomeric carbon. Anomerization is the process of conversion of one anomer to the other...
-glycosidic linkage
Glycosidic bond
In chemistry, a glycosidic bond is a type of covalent bond that joins a carbohydrate molecule to another group, which may or may not be another carbohydrate....
. Examples of nucleosides include cytidine
Cytidine
Cytidine is a nucleoside molecule that is formed when cytosine is attached to a ribose ring via a β-N1-glycosidic bond...
, uridine
Uridine
Uridine is a molecule that is formed when uracil is attached to a ribose ring via a β-N1-glycosidic bond.If uracil is attached to a deoxyribose ring, it is known as a deoxyuridine....
, adenosine, guanosine
Guanosine
Guanosine is a purine nucleoside comprising guanine attached to a ribose ring via a β-N9-glycosidic bond. Guanosine can be phosphorylated to become guanosine monophosphate , cyclic guanosine monophosphate , guanosine diphosphate , and guanosine triphosphate...
, thymidine
Thymidine
Thymidine is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine in double-stranded DNA...
and inosine
Inosine
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring via a β-N9-glycosidic bond....
.
Biological function
Nucleosides can be phosphorylatedPhosphorylation
Phosphorylation is the addition of a phosphate group to a protein or other organic molecule. Phosphorylation activates or deactivates many protein enzymes....
by specific kinase
Kinase
In chemistry and biochemistry, a kinase is a type of enzyme that transfers phosphate groups from high-energy donor molecules, such as ATP, to specific substrates, a process referred to as phosphorylation. Kinases are part of the larger family of phosphotransferases...
s in the cell on the sugar's primary alcohol group (-CH2-OH), producing nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
s, which are the molecular building-blocks of DNA
DNA
Deoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
and RNA
RNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
.
Nucleosides can be produced by de novo synthesis
De novo synthesis
De novo synthesis refers to the synthesis of complex molecules from simple molecules such as sugars or amino acids, as opposed to their being recycled after partial degradation. For example, nucleotides are not needed in the diet as they can be constructed from small precursor molecules such as...
pathways, in particular in the liver, but they are more abundantly supplied via ingestion and digestion of nucleic acids in the diet, whereby nucleotidase
Nucleotidase
A nucleotidase is a hydrolytic enzyme that catalyzes the hydrolysis of a nucleotide into a nucleoside and a phosphate. For example, they convert adenosine monophosphate to adenosine, and guanosine monophosphate to guanosine....
s break down nucleotide
Nucleotide
Nucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
s (such as the thymidine monophosphate
Thymidine monophosphate
Thymidine monophosphate, also known as 5'-thymidylate, thymidylate, or TMP, is a nucleotide that is used as a monomer in DNA. It is an ester of phosphoric acid with the nucleoside thymidine. TMP consists of a phosphate group, the pentose sugar deoxyribose, and the nucleobase thymine...
) into nucleosides (such as thymidine
Thymidine
Thymidine is a chemical compound, more precisely a pyrimidine deoxynucleoside. Deoxythymidine is the DNA nucleoside T, which pairs with deoxyadenosine in double-stranded DNA...
) and phosphate. The nucleosides, in turn, are subsequently broken down:
- in the lumenLumen (anatomy)A lumen in biology is the inside space of a tubular structure, such as an artery or intestine...
of the digestive system by nucleosidases into nucleobases and ribose or deoxyribose.
In addition, nucleotides can be broken down:
- inside the cell into nitrogenous baseNitrogenous baseA nitrogenous base is a nitrogen-containing molecule having the chemical properties of a base. It is an organic compound that owes its property as a base to the lone pair of electrons of a nitrogen atom. In biological sciences, nitrogenous bases are typically classified as the derivatives of two...
s, and ribose-1-phosphate or deoxyribose-1-phosphate.
Use in medicine and technology
In medicine several nucleoside analoguesNucleoside analogues
Nucleoside analogues are a range of antiviral products used to prevent viral replication in infected cells. The most commonly used is Acyclovir, although its inclusion in this category is uncertain, as it contains only a partial nucleoside structure, as the sugar ring is replaced by an open-chain...
are used as antiviral or anticancer agents. The viral polymerase incorporates these compounds with non-canonical bases. These compounds are activated in the cells by being converted into nucleotides, they are administered as nucleosides since charged nucleotides cannot easily cross cell membranes.
In molecular biology, several analogues
Nucleic acid analogues
Nucleic acid analogues are compounds structurally similar to naturally occurring RNA and DNA, used in medicine and in molecular biology research....
of the sugar backbone exist. Due to the low stability of RNA, which is prone to hydrolysis, several more stable alternative nucleoside/nucleotide analogues that correctly bind to RNA are used. This is achieved by using a different backbone sugar. These analogues include LNA
Locked nucleic acid
A locked nucleic acid , often referred to as inaccessible RNA, is a modified RNA nucleotide. The ribose moiety of an LNA nucleotide is modified with an extra bridge connecting the 2' oxygen and 4' carbon. The bridge "locks" the ribose in the 3'-endo conformation, which is often found in the A-form...
, morpholino
Morpholino
In molecular biology, a Morpholino is a molecule in a particular structural family that is used to modify gene expression. Morpholino oligomers are an antisense technology used to block access of other molecules to specific sequences within nucleic acid...
, PNA.
In sequencing, dideoxynucleotides are used. These nucleotides possess the non-canon sugar dideoxyribose, which lacks 3' hydroxyl group (which accepts the phosphate) and therefore cannot bond with the next base, terminating the chain, as DNA polymerases cannot distinguish between it and a regular deoxyribonucleotide.
See also
- Adenosine triphosphateAdenosine triphosphateAdenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP) - NucleotideNucleotideNucleotides are molecules that, when joined together, make up the structural units of RNA and DNA. In addition, nucleotides participate in cellular signaling , and are incorporated into important cofactors of enzymatic reactions...
- NucleobaseNucleobaseNucleobases are a group of nitrogen-based molecules that are required to form nucleotides, the basic building blocks of DNA and RNA. Nucleobases provide the molecular structure necessary for the hydrogen bonding of complementary DNA and RNA strands, and are key components in the formation of stable...
- Synthesis of nucleosidesSynthesis of nucleosidesSynthesis of nucleosides involves the coupling of a nucleophilic, heterocyclic base with an electrophilic sugar. The silyl-Hilbert-Johnson reaction, which employs silylated heterocyclic bases and electrophilic sugar derivatives in the presence of a Lewis acid, is the most common method for forming...

