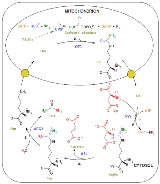
Urea cycle
Encyclopedia
The urea cycle is a cycle of biochemical
reactions occurring in many animals that produces urea
((N
H
2)2C
O
) from ammonia
(NH3). This cycle was the first metabolic cycle discovered (Hans Krebs
and Kurt Henseleit, 1932), five years before the discovery of the TCA cycle. In mammals, the urea cycle takes place primarily in the liver
, and to a lesser extent in the kidney
.
or uric acid
, which are much less toxic. Insufficiency of the urea cycle occurs in some genetic disorder
s (inborn errors of metabolism
), and in liver failure
. The result of liver failure is accumulation of nitrogenous waste, mainly ammonia, which leads to hepatic encephalopathy
.
ic. The cycle converts two amino groups, one from NH4+ and one from Asp
, and a carbon atom from HCO3−, to the relatively nontoxic excretion product urea
at the cost of four "high-energy" phosphate bonds (3 ATP hydrolyzed to 2 ADP and one AMP). Ornithine
is the carrier of these carbon and nitrogen atoms.
|+ Reactions of the urea cycle
! Step > Reactants
Products
Catalyzed by
! 1
| NH4+ + HCO3− + 2ATP
carbamoyl phosphate
+ 2ADP
+ Pi
CPS1
! 2
| carbamoyl phosphate
+ ornithine
citrulline
+ Pi
OTC
! 3
| citrulline
+ aspartate
+ ATP
argininosuccinate + AMP
+ PPi
ASS
! 4
| argininosuccinate Arg
+ fumarate
ASL
! 5
| Arg
+ H2O ornithine
+ urea
ARG1
cytosol
In the first reaction, NH4+ + HCO3− is equivalent to NH3
+ CO2
+ H2O
.
Thus, the overall equation of the urea cycle is:
Since fumarate is obtained by removing NH3 from aspartate (by means of reactions 3 and 4), and PPi + H2O → 2 Pi, the equation can be simplified as follows:
Note that reactions related to the urea cycle also cause the oxidation of 2 NADH, so the urea cycle releases slightly more energy than it consumes. These NADH are produced in two ways:
The two NADH produced can provide energy for the formation of 5 ATP
, a net production of one high-energy phosphate bond for the urea cycle. However, if gluconeogenesis
is underway in the cytosol, the latter reducing equivalent is used to drive the reversal of the GAPDH step instead of generating ATP.
The fate of oxaloacetate is either to produce aspartate via transamination or to be converted to phosphoenol pyruvate, which is a substrate to glucose.
The anomalous substrate buildup is not without cost, however. The substrate concentrations become elevated all the way back up the cycle to NH4+, resulting in hyperammonemia
(elevated [NH4+]P).
Although the root cause of NH4+ toxicity is not completely understood, a high [NH4+] puts an enormous strain on the NH4+-clearing system, especially in the brain
(symptoms of urea cycle enzyme deficiencies include mental retardation
and lethargy). This clearing system involves GLUD1 and GLUL, which decrease the 2-oxoglutarate (2OG) and Glu pools. The brain is most sensitive to the depletion of these pools. Depletion of 2OG decreases the rate of TCAC, whereas Glu is both a neurotransmitter
and a precursor to GABA
, another neurotransmitter. http://www.wiley.com/college/math/chem/cg/sales/voet.html(p.734)
Most of them are associated with hyperammonemia
.
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
reactions occurring in many animals that produces urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
((N
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
H
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
2)2C
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
O
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
) from ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
(NH3). This cycle was the first metabolic cycle discovered (Hans Krebs
Hans Adolf Krebs
Sir Hans Adolf Krebs was a German-born British physician and biochemist. Krebs is best known for his identification of two important metabolic cycles: the urea cycle and the citric acid cycle...
and Kurt Henseleit, 1932), five years before the discovery of the TCA cycle. In mammals, the urea cycle takes place primarily in the liver
Liver
The liver is a vital organ present in vertebrates and some other animals. It has a wide range of functions, including detoxification, protein synthesis, and production of biochemicals necessary for digestion...
, and to a lesser extent in the kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
.
Function
Organisms that cannot easily and quickly remove ammonia usually have to convert it to some other substance, like ureaUrea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
or uric acid
Uric acid
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates such as ammonium acid urate. Uric acid is created when the body breaks down purine nucleotides. High blood concentrations of uric acid...
, which are much less toxic. Insufficiency of the urea cycle occurs in some genetic disorder
Genetic disorder
A genetic disorder is an illness caused by abnormalities in genes or chromosomes, especially a condition that is present from before birth. Most genetic disorders are quite rare and affect one person in every several thousands or millions....
s (inborn errors of metabolism
Inborn error of metabolism
Inborn errors of metabolism comprise a large class of genetic diseases involving disorders of metabolism. The majority are due to defects of single genes that code for enzymes that facilitate conversion of various substances into others...
), and in liver failure
Liver failure
Acute liver failure is the appearance of severe complications rapidly after the first signs of liver disease , and indicates that the liver has sustained severe damage . The complications are hepatic encephalopathy and impaired protein synthesis...
. The result of liver failure is accumulation of nitrogenous waste, mainly ammonia, which leads to hepatic encephalopathy
Hepatic encephalopathy
Hepatic encephalopathy is the occurrence of confusion, altered level of consciousness and coma as a result of liver failure. In the advanced stages it is called hepatic coma or coma hepaticum...
.
Reactions
The urea cycle consists of five reactions: two mitochondrial and three cytosolCytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
ic. The cycle converts two amino groups, one from NH4+ and one from Asp
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
, and a carbon atom from HCO3−, to the relatively nontoxic excretion product urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
at the cost of four "high-energy" phosphate bonds (3 ATP hydrolyzed to 2 ADP and one AMP). Ornithine
Ornithine
Ornithine is an amino acid that plays a role in the urea cycle.-Role in urea cycle:L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen....
is the carrier of these carbon and nitrogen atoms.
! Step >
! 1
| NH4+ + HCO3− + 2ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
Carbamoyl phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals it is an intermediary metabolite participating in the nitrogen disposal through in the urea cycle and the synthesis of pyrimidines....
+ 2ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
+ Pi
! 2
| carbamoyl phosphate
Carbamoyl phosphate
Carbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals it is an intermediary metabolite participating in the nitrogen disposal through in the urea cycle and the synthesis of pyrimidines....
+ ornithine
Ornithine
Ornithine is an amino acid that plays a role in the urea cycle.-Role in urea cycle:L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen....
Citrulline
The organic compound citrulline is an α-amino acid. Its name is derived from citrullus, the Latin word for watermelon, from which it was first isolated in 1930.It has the idealized formula H2NCNH3CHCO2H...
+ Pi
! 3
| citrulline
Citrulline
The organic compound citrulline is an α-amino acid. Its name is derived from citrullus, the Latin word for watermelon, from which it was first isolated in 1930.It has the idealized formula H2NCNH3CHCO2H...
+ aspartate
Aspartic acid
Aspartic acid is an α-amino acid with the chemical formula HOOCCHCH2COOH. The carboxylate anion, salt, or ester of aspartic acid is known as aspartate. The L-isomer of aspartate is one of the 20 proteinogenic amino acids, i.e., the building blocks of proteins...
+ ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
Adenosine monophosphate
Adenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
+ PPi
Pyrophosphate
In chemistry, the anion, the salts, and the esters of pyrophosphoric acid are called pyrophosphates. Any salt or ester containing two phosphate groups is called a diphosphate. As a food additive, diphosphates are known as E450.- Chemistry :...
Argininosuccinate synthetase
Argininosuccinate synthase is an enzyme that catalyzes the synthesis of argininosuccinate from citrulline and aspartateASS is responsible for the third step of the urea cycle and one of the reactions of the Citrulline-NO cycle.-Gene:...
! 4
| argininosuccinate
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
+ fumarate
! 5
| Arg
Arginine
Arginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
+ H2O
Ornithine
Ornithine is an amino acid that plays a role in the urea cycle.-Role in urea cycle:L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen....
+ urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
- The reactions of the urea cycle
1 L-ornithineOrnithineOrnithine is an amino acid that plays a role in the urea cycle.-Role in urea cycle:L-Ornithine is one of the products of the action of the enzyme arginase on L-arginine, creating urea. Therefore, ornithine is a central part of the urea cycle, which allows for the disposal of excess nitrogen....
2 carbamoyl phosphateCarbamoyl phosphateCarbamoyl phosphate is an anion of biochemical significance. In land-dwelling animals it is an intermediary metabolite participating in the nitrogen disposal through in the urea cycle and the synthesis of pyrimidines....
3 L-citrullineCitrullineThe organic compound citrulline is an α-amino acid. Its name is derived from citrullus, the Latin word for watermelon, from which it was first isolated in 1930.It has the idealized formula H2NCNH3CHCO2H...
4 argininosuccinate
5 fumarate
6 L-arginineArginineArginine is an α-amino acid. The L-form is one of the 20 most common natural amino acids. At the level of molecular genetics, in the structure of the messenger ribonucleic acid mRNA, CGU, CGC, CGA, CGG, AGA, and AGG, are the triplets of nucleotide bases or codons that codify for arginine during...
7 ureaUreaUrea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
L-Asp L-aspartate
CPS-1 carbamoyl phosphate synthetase ICarbamoyl phosphate synthetase ICarbamoyl Phosphate Synthetase I is a ligase enzyme located in the mitochondria involved in the production of urea. Carbamoyl Phosphate Synthetase I transfers an ammonia from glutamine to a molecule of bicarbonate that has been phosphorylated by a molecule of ATP. The resulting carbamate is...
OTC Ornithine transcarbamoylase
ASS argininosuccinate synthetaseArgininosuccinate synthetaseArgininosuccinate synthase is an enzyme that catalyzes the synthesis of argininosuccinate from citrulline and aspartateASS is responsible for the third step of the urea cycle and one of the reactions of the Citrulline-NO cycle.-Gene:...
ASL argininosuccinate lyase
ARG1 arginase 1ArginaseArginase is a manganese-containing enzyme. The reaction catalyzed by this enzyme is: arginine + H2O → ornithine + urea. It is the final enzyme of the urea cycle.- Structure and function :Arginase belong to the ureohydrolase family of enzymes....
In the first reaction, NH4+ + HCO3− is equivalent to NH3
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
+ CO2
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
+ H2O
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
.
Thus, the overall equation of the urea cycle is:
- NH3AmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
+ CO2Carbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
+ aspartate + 3 ATPAdenosine triphosphateAdenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
+ 2 H2OWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
→ ureaUreaUrea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
+ fumarate + 2 ADPAdenosine diphosphateAdenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
+ 2 PiPhosphateA phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
+ AMPAdenosine monophosphateAdenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
+ PPiPyrophosphateIn chemistry, the anion, the salts, and the esters of pyrophosphoric acid are called pyrophosphates. Any salt or ester containing two phosphate groups is called a diphosphate. As a food additive, diphosphates are known as E450.- Chemistry :...
Since fumarate is obtained by removing NH3 from aspartate (by means of reactions 3 and 4), and PPi + H2O → 2 Pi, the equation can be simplified as follows:
- 2 NH3AmmoniaAmmonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
+ CO2Carbon dioxideCarbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
+ 3 ATPAdenosine triphosphateAdenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
+ H2OWaterWater is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
→ ureaUreaUrea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
+ 2 ADPAdenosine diphosphateAdenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
+ 4 PiPhosphateA phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
+ AMPAdenosine monophosphateAdenosine monophosphate , also known as 5'-adenylic acid, is a nucleotide that is used as a monomer in RNA. It is an ester of phosphoric acid and the nucleoside adenosine. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine...
Note that reactions related to the urea cycle also cause the oxidation of 2 NADH, so the urea cycle releases slightly more energy than it consumes. These NADH are produced in two ways:
- One NADH molecule is reduced by the enzyme glutamate dehydrogenaseGlutamate dehydrogenaseGlutamate dehydrogenase is an enzyme, present in most microbes and the mitochondria of eukaryotes, as are some of the other enzymes required for urea synthesis, that converts glutamate to α-Ketoglutarate, and vice versa. In animals, the produced ammonia is, however, usually bled off to the urea...
in the conversion of glutamate to ammonium and α-ketoglutarate. Glutamate is the non-toxic carrier of amine groups. This provides the ammonium ion used in the initial synthesis of carbamoyl phosphate. - The fumarate released in the cytosol is converted to malateMalateMalate is the ionized form of malic acid. It is an important chemical compound in biochemistry. In the C4 carbon fixation process, malate is a source of CO2 in the Calvin cycle....
by cytosolic fumaraseFumaraseFumarase is an enzyme that catalyzes the reversible hydration/dehydration of Fumarate to malate. Fumarase comes in two forms: mitochondrial and cytosolic...
. This malate is then converted to oxaloacetate by cytosolic malate dehydrogenaseMalate dehydrogenaseMalate dehydrogenase is an enzyme in the citric acid cycle that catalyzes the conversion of malate into oxaloacetate and vice versa...
, generating a reduced NADH in the cytosol. Oxaloacetate is one of the keto acids preferred by transaminaseTransaminaseIn biochemistry, a transaminase or an aminotransferase is an enzyme that catalyzes a type of reaction between an amino acid and an α-keto acid. To be specific, this reaction involves removing the amino group from the amino acid, leaving behind an α-keto acid, and transferring it to the...
s, and so will be recycled to aspartate, maintained the flow of nitrogen into the urea cycle.
The two NADH produced can provide energy for the formation of 5 ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
, a net production of one high-energy phosphate bond for the urea cycle. However, if gluconeogenesis
Gluconeogenesis
Gluconeogenesis is a metabolic pathway that results in the generation of glucose from non-carbohydrate carbon substrates such as lactate, glycerol, and glucogenic amino acids....
is underway in the cytosol, the latter reducing equivalent is used to drive the reversal of the GAPDH step instead of generating ATP.
The fate of oxaloacetate is either to produce aspartate via transamination or to be converted to phosphoenol pyruvate, which is a substrate to glucose.
N-Acetylglutamic acid
The synthesis of carbamoyl phosphate and the urea cycle are dependent on the presence of NAcGlu, which allosterically activates CPS1. Synthesis of NAcGlu by NAGS, is stimulated by both Arg, allosteric stimulator of NAGS, and Glu, a product in the transamination reactions and one of NAGS's substrates, both of which are elevated when free amino acids are elevated. So, Arg is not only a substrate for the urea cycle reactions but also serves as an activator for the urea cycle.Substrate concentrations
The remaining enzymes of the cycle are controlled by the concentrations of their substrates. Thus, inherited deficiencies in the cycle enzymes other than ARG1 do not result in significant decrease in urea production (the total lack of any cycle enzyme results in death shortly after birth). Rather, the deficient enzyme's substrate builds up, increasing the rate of the deficient reaction to normal.The anomalous substrate buildup is not without cost, however. The substrate concentrations become elevated all the way back up the cycle to NH4+, resulting in hyperammonemia
Hyperammonemia
Hyperammonemia is a metabolic disturbance characterised by an excess of ammonia in the blood. It is a dangerous condition that may lead to encephalopathy and death. It may be primary or secondary....
(elevated [NH4+]P).
Although the root cause of NH4+ toxicity is not completely understood, a high [NH4+] puts an enormous strain on the NH4+-clearing system, especially in the brain
Human brain
The human brain has the same general structure as the brains of other mammals, but is over three times larger than the brain of a typical mammal with an equivalent body size. Estimates for the number of neurons in the human brain range from 80 to 120 billion...
(symptoms of urea cycle enzyme deficiencies include mental retardation
Mental retardation
Mental retardation is a generalized disorder appearing before adulthood, characterized by significantly impaired cognitive functioning and deficits in two or more adaptive behaviors...
and lethargy). This clearing system involves GLUD1 and GLUL, which decrease the 2-oxoglutarate (2OG) and Glu pools. The brain is most sensitive to the depletion of these pools. Depletion of 2OG decreases the rate of TCAC, whereas Glu is both a neurotransmitter
Neurotransmitter
Neurotransmitters are endogenous chemicals that transmit signals from a neuron to a target cell across a synapse. Neurotransmitters are packaged into synaptic vesicles clustered beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to...
and a precursor to GABA
Gabâ
Gabâ or gabaa, for the people in many parts of the Philippines), is the concept of a non-human and non-divine, imminent retribution. A sort of negative karma, it is generally seen as an evil effect on a person because of their wrongdoings or transgressions...
, another neurotransmitter. http://www.wiley.com/college/math/chem/cg/sales/voet.html(p.734)
Pathology
Anomalies of the urea cycle cause urea cycle disorders:- ornithine transcarbamoylase deficiency
- Carbamoyl phosphate synthetase deficiency (Ornithine translocase deficiencyOrnithine translocase deficiencyOrnithine translocase deficiency, also called Hyperornithinemia-Hyperammonemia-Homocitrullinuria syndrome, is a rare autosomal recessive urea cycle disorder affecting the enzyme ornithine translocase, which causes ammonia to accumulate in the blood, a condition called hyperammonemia.Ammonia, which...
) - Argininosuccinic aciduriaArgininosuccinic aciduriaArgininosuccinic aciduria, also called argininosuccinic acidemia, is an inherited disorder that causes the accumulation of argininosuccinic acid in the blood and urine. Some patients may also have an elevation of ammonia, a toxic chemical, which can affect the nervous system...
- ArgininemiaArgininemiaArgininemia, also called arginase deficiency, is an autosomal recessive urea cycle disorder where a deficiency of the enzyme arginase causes a build up of arginine and ammonia in the blood....
- Hyperornithinemia, hyperammonemia, homocitrullinuria syndrome (HHH syndrome)
- Lysinuric protein intoleranceLysinuric protein intoleranceLysinuric protein intolerance , also called hyperdibasic aminoaciduria type 2 or familial protein intolerance, is an autosomal recessive metabolic disorder affecting amino acid transport....
- CitrullinemiaCitrullinemiaCitrullinemia, also called citrullinuria, is an autosomal recessive urea cycle disorder that causes ammonia and other toxic substances to accumulate in the blood....
- N-Acetylglutamate synthase deficiency
Most of them are associated with hyperammonemia
Hyperammonemia
Hyperammonemia is a metabolic disturbance characterised by an excess of ammonia in the blood. It is a dangerous condition that may lead to encephalopathy and death. It may be primary or secondary....
.
External links
- The chemical logic behind the urea cycle
- Basic Neurochemistry - amino acid disorders

