
Glutamate dehydrogenase
Encyclopedia
Glutamate dehydrogenase (GLDH) is an enzyme
, present in most microbes and the mitochondria of eukaryotes, as are some of the other enzymes required for urea
synthesis, that converts glutamate to α-Ketoglutarate, and vice versa. In animals, the produced ammonia is, however, usually bled off to the urea cycle
. In bacteria, the ammonia is assimilated to amino acids via glutamate and amidotransferases. In plants, the enzyme can work in either direction depending on environment and stress. The enzyme represents a key link between catabolic and metabolic pathways, and is, therefore, ubiquitous in eukaryotes.
to evaluate the liver function. Elevated blood serum
GLDH levels indicate liver damage and GLDH plays an important role in the differential diagnosis of liver disease, especially in combination with aminotransferases. GLDH is localised in mitochondria, therefore practically none is liberated in generalised inflammatory diseases of the liver such as viral hepatitides. Liver diseases in which necrosis of hepatocytes is the predominant event, such as toxic liver damage or hypoxic liver disease, are characterised by high serum GLDH levels. GLDH is important for distinguishing between acute viral hepatitis and acute toxic liver necrosis or acute hypoxic liver disease, particularly in the case of liver damage with very high aminotransferases. In clinical trials, GLDH can serve as a measurement for the safety of a drug.
+(or NADP+) is a cofactor
for the glutamate dehydrogenase reaction, producing α-Ketoglutarate and ammonium
as a byproduct.
Based on which cofactor is used, glutamate dehydrogenase enzymes are divided into the following three classes:
. Glutamate plays the central role in mammalian and microbe nitrogen flow, serving as both a nitrogen donor and a nitrogen acceptor.
. This regulation is relaxed in response to caloric restriction and low blood glucose. Under these circumstances, glutamate dehydrogenase activity is raised in order to increase the amount of α-Ketoglutarate produced, which can be used to provide energy by being used in the citric acid cycle
to ultimately produce ATP
.
In microbes, the activity is controlled by the concentration of ammonium and or the like-sized Rubidium ion, which binds to an allosteric site on GDH and change the Km (Michaelis constant) of the enzyme.
The control of GDH through ADP-ribosylation is particularly important in insulin
-producing β cells. Beta cells secrete insulin in response to an increase in the ATP:ADP
ratio, and, as amino acids are broken down by GDH into α-ketoglutarate, this ratio rises and more insulin is secreted. SIRT4 is necessary to regulate the metabolism of amino acids as a method of controlling insulin secretion and regulating blood glucose
levels.
:
Activators:
s:
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
, present in most microbes and the mitochondria of eukaryotes, as are some of the other enzymes required for urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
synthesis, that converts glutamate to α-Ketoglutarate, and vice versa. In animals, the produced ammonia is, however, usually bled off to the urea cycle
Urea cycle
The urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia . This cycle was the first metabolic cycle discovered , five years before the discovery of the TCA cycle...
. In bacteria, the ammonia is assimilated to amino acids via glutamate and amidotransferases. In plants, the enzyme can work in either direction depending on environment and stress. The enzyme represents a key link between catabolic and metabolic pathways, and is, therefore, ubiquitous in eukaryotes.
Clinical application
GLDH can be measured in a medical laboratoryMedical laboratory
A medical laboratory or clinical laboratory is a laboratory where tests are done on clinical specimens in order to get information about the health of a patient as pertaining to the diagnosis, treatment, and prevention of disease.-Departments:...
to evaluate the liver function. Elevated blood serum
Blood serum
In blood, the serum is the component that is neither a blood cell nor a clotting factor; it is the blood plasma with the fibrinogens removed...
GLDH levels indicate liver damage and GLDH plays an important role in the differential diagnosis of liver disease, especially in combination with aminotransferases. GLDH is localised in mitochondria, therefore practically none is liberated in generalised inflammatory diseases of the liver such as viral hepatitides. Liver diseases in which necrosis of hepatocytes is the predominant event, such as toxic liver damage or hypoxic liver disease, are characterised by high serum GLDH levels. GLDH is important for distinguishing between acute viral hepatitis and acute toxic liver necrosis or acute hypoxic liver disease, particularly in the case of liver damage with very high aminotransferases. In clinical trials, GLDH can serve as a measurement for the safety of a drug.
Cofactors
NADNicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide, abbreviated NAD, is a coenzyme found in all living cells. The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine base and the other nicotinamide.In metabolism, NAD is involved...
+(or NADP+) is a cofactor
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
for the glutamate dehydrogenase reaction, producing α-Ketoglutarate and ammonium
Ammonium
The ammonium cation is a positively charged polyatomic cation with the chemical formula NH. It is formed by the protonation of ammonia...
as a byproduct.
Based on which cofactor is used, glutamate dehydrogenase enzymes are divided into the following three classes:
- EC 1.4.1.2: L-glutamate + H2O + NAD+
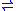 2-oxoglutarate + NH3 + NADH + H+
2-oxoglutarate + NH3 + NADH + H+ - EC 1.4.1.3: L-glutamate + H2O + NAD(P)+
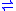 2-oxoglutarate + NH3 + NAD(P)H + H+
2-oxoglutarate + NH3 + NAD(P)H + H+ - EC 1.4.1.4: L-glutamate + H2O + NADP+
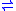 2-oxoglutarate + NH3 + NADPH + H+
2-oxoglutarate + NH3 + NADPH + H+
Role in flow of nitrogen
Ammonia incorporation in animals and microbes occurs through the actions of glutamate dehydrogenase and glutamine synthetaseGlutamine synthetase
Glutamine synthetase is an enzyme that plays an essential role in the metabolism of nitrogen by catalyzing the condensation of glutamate and ammonia to form glutamine:Glutamate + ATP + NH3 → Glutamine + ADP + phosphate...
. Glutamate plays the central role in mammalian and microbe nitrogen flow, serving as both a nitrogen donor and a nitrogen acceptor.
Regulation of glutamate dehydrogenase
In Humans, the activity of glutamate dehydrogenase is controlled through ADP-ribosylation, a covalent modification carried out by the gene sirt4SIRT4
Sirtuin 4, also known as SIRT4 is a protein which in humans is encoded by the SIRT4 gene.-Function:This gene encodes a member of the sirtuin family of proteins which are homologs of the Sir2 gene in budding yeast. Members of the sirtuin family are characterized by a sirtuin core domain and grouped...
. This regulation is relaxed in response to caloric restriction and low blood glucose. Under these circumstances, glutamate dehydrogenase activity is raised in order to increase the amount of α-Ketoglutarate produced, which can be used to provide energy by being used in the citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
to ultimately produce ATP
Adenosine triphosphate
Adenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
.
In microbes, the activity is controlled by the concentration of ammonium and or the like-sized Rubidium ion, which binds to an allosteric site on GDH and change the Km (Michaelis constant) of the enzyme.
The control of GDH through ADP-ribosylation is particularly important in insulin
Insulin
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
-producing β cells. Beta cells secrete insulin in response to an increase in the ATP:ADP
Adenosine diphosphate
Adenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
ratio, and, as amino acids are broken down by GDH into α-ketoglutarate, this ratio rises and more insulin is secreted. SIRT4 is necessary to regulate the metabolism of amino acids as a method of controlling insulin secretion and regulating blood glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
levels.
Regulation
Allosteric inhibitorsAllosteric regulation
In biochemistry, allosteric regulation is the regulation of an enzyme or other protein by binding an effector molecule at the protein's allosteric site . Effectors that enhance the protein's activity are referred to as allosteric activators, whereas those that decrease the protein's activity are...
:
- Adenosine triphosphateAdenosine triphosphateAdenosine-5'-triphosphate is a multifunctional nucleoside triphosphate used in cells as a coenzyme. It is often called the "molecular unit of currency" of intracellular energy transfer. ATP transports chemical energy within cells for metabolism...
(ATP) - Guanosine triphosphateGuanosine triphosphateGuanosine-5'-triphosphate is a purine nucleoside triphosphate. It can act as a substrate for the synthesis of RNA during the transcription process...
(GTP)
Activators:
- Adenosine diphosphateAdenosine diphosphateAdenosine diphosphate, abbreviated ADP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside adenosine. ADP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase adenine....
(ADP) - Guanosine diphosphateGuanosine diphosphateGuanosine diphosphate, abbreviated GDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside guanosine. GDP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase guanine....
(GDP)
Isozymes
Humans express the following glutamate dehydrogenase isozymeIsozyme
Isozymes are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These enzymes usually display different kinetic parameters Isozymes (also known as isoenzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. These enzymes...
s:

