Fumarase
Encyclopedia
Fumarase is an enzyme
that catalyzes the reversible hydration
/dehydration
of Fumarate to malate
. Fumarase comes in two forms: mitochondrial and cytosolic. The mitochondrial isoenzyme is involved in the Krebs Cycle (also known as the Tricarboxylic Acid Cycle [TCA] or the Citric Acid Cycle), and the cytosolic isoenzyme is involved in the metabolism
of amino acids and fumarate. Subcellular localization is established by the presence of a signal sequence on the amino terminus in the mitochondrial form, while subcellular localization in the cytosolic form is established by the absence of the signal sequence found in the mitochondrial variety.
This enzyme participates in 3 metabolic pathways
: citric acid cycle
, reductive citric acid cycle (CO2 fixation), and in renal cell carcinoma
.
s, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme class is (S)-malate hydro-lyase (fumarate-forming). Other names in common use include:

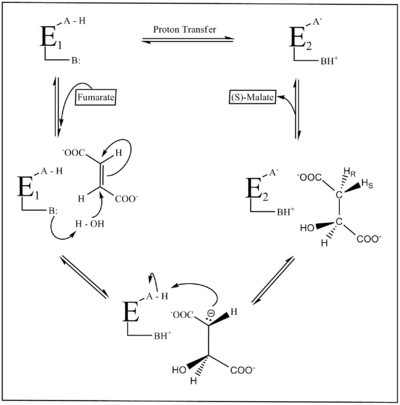 Figure 2 depicts the fumarase reaction mechanism. Two acid-base groups catalyze proton transfer, and the ionization state of these groups is in part defined by two forms of the enzyme E1 and E2. In E1, the groups exist in an internally neutralized A-H/B: state, while in E2, they occur in a zwitterion
Figure 2 depicts the fumarase reaction mechanism. Two acid-base groups catalyze proton transfer, and the ionization state of these groups is in part defined by two forms of the enzyme E1 and E2. In E1, the groups exist in an internally neutralized A-H/B: state, while in E2, they occur in a zwitterion
ic A-/BH+ state. E1 binds fumarate and facilitates its tansformation into malate, and E2 binds malate and facilitates its transformation into fumarate. The two forms must undergo isomerization with each catalytic turnover.
Despite its biological significance, the reaction mechanism of fumarase is not completely understood. The reaction itself can be monitored in either direction; however, it is the formation of fumarate from S-malate in particular that is less understood due to the high pKa
value of the HR (Fig. 1) atom that is removed without the aid of any cofactors
or coenzymes. However, the reaction from fumarate to L-malate is better understood, and involves a stereospecific hydration of fumarate to produce S-malate by trans-addition of a hydroxyl
group and a hydrogen atom through a trans 1,4 addition of a hydroxyl group. Early research into this reaction suggested that the formation of fumarate from S-malate involved dehydration of malate to a carbocationic intermediate, which then loses the alpha proton to form fumarate. This led to the conclusion that in the formation of S-Malate from fumarate E1 elimination, protonation of fumarate to the carbocation was followed by the additional of a hydroxyl group from H2O. However, more recent trials have provided evidence that the mechanism actually takes place through an acid-base catalyzed elimination by means of a carbanionic intermediate E1CB elimination
(Figure 2).
is to facilitate a transition step in the production of energy in the form of NADH. In the cytosol
the enzyme functions to metabolize fumarate, which ends up as a byproduct of the urea cycle
as well as amino acid catabolism. Studies have revealed that the active site is composed of amino acid residues from three of the four subunits within the tetrameric enzyme.
The primary binding site on fumarase is known as catalytic site A. Studies have revealed that catalytic site A is composed of amino acid residues from three of the four subunits within the tetrameric enzyme. Two potential acid-base catalytic residues in the reaction include His 188 and Lys 324.
is characterized by polyhydramnios
and fetal brain abnormalities. In the newborn period, findings include severe neurologic abnormalities, poor feeding, failure to thrive, and hypotonia
. Fumarase deficiency is suspected in infants with multiple severe neurologic abnormalities in the absence of an acute metabolic crisis. Inactivity of both cytosolic and mitochondrial forms of fumarase are potential causes. Isolated, increased concentration of fumaric acid
on urine organic acid analysis is highly suggestive of fumarase deficiency. Molecular genetic testing for fumarase deficiency is currently available.
Fumarase is prevalent in both fetal and adult tissues. A large percentage of the enzyme is expressed in the skin
, parathyroid, lymph
, and colon
. Mutations in the production and development of fumarase have led to the discovery of several fumarase-related diseases in humans. These include benign
mesenchymal tumors of the uterus, leiomyomatosis and renal cell carcinoma
, and fumarase deficiency
. Germinal mutations in fumarase are associated with two distinct conditions. If the enzyme has missense mutation and in-frame deletions from the 3’ end, fumarase deficiency results. If it contains heterozygous 5’ missense mutation and deletions (ranging from one base pair to the whole gene), then leiomyomatosis and renal cell carcinoma/Reed’s syndrome (multiple cutaneous
and uterine leiomyomatosis) could result.
have been observed to have two occupied dicarboxylate binding sites. These are known as the active site
and the B site. The active site and B site are both identified as having areas unoccupied by a bound ligand
. This so-called ‘free’ crystal structure demonstrates conservation of the active-site water. Similar orientation has been discovered in other fumarase C crystal structures. Crystallographic research on the B site of the enzyme has observed that there is a shift on His129. This information suggests that water is a permanent component of the active site. It also suggests that the use of an imidazole
-imidazolium conversion controls access to the allosteric B site.
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
that catalyzes the reversible hydration
Hydration reaction
In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous...
/dehydration
Dehydration
In physiology and medicine, dehydration is defined as the excessive loss of body fluid. It is literally the removal of water from an object; however, in physiological terms, it entails a deficiency of fluid within an organism...
of Fumarate to malate
Malate
Malate is the ionized form of malic acid. It is an important chemical compound in biochemistry. In the C4 carbon fixation process, malate is a source of CO2 in the Calvin cycle....
. Fumarase comes in two forms: mitochondrial and cytosolic. The mitochondrial isoenzyme is involved in the Krebs Cycle (also known as the Tricarboxylic Acid Cycle [TCA] or the Citric Acid Cycle), and the cytosolic isoenzyme is involved in the metabolism
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
of amino acids and fumarate. Subcellular localization is established by the presence of a signal sequence on the amino terminus in the mitochondrial form, while subcellular localization in the cytosolic form is established by the absence of the signal sequence found in the mitochondrial variety.
This enzyme participates in 3 metabolic pathways
Metabolism
Metabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
: citric acid cycle
Citric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
, reductive citric acid cycle (CO2 fixation), and in renal cell carcinoma
Renal cell carcinoma
Renal cell carcinoma is a kidney cancer that originates in the lining of the proximal convoluted tubule, the very small tubes in the kidney that filter the blood and remove waste products. RCC is the most common type of kidney cancer in adults, responsible for approximately 80% of cases...
.
Nomenclature
This enzyme belongs to the family of lyaseLyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking of various chemical bonds by means other than hydrolysis and oxidation, often forming a new double bond or a new ring structure...
s, specifically the hydro-lyases, which cleave carbon-oxygen bonds. The systematic name of this enzyme class is (S)-malate hydro-lyase (fumarate-forming). Other names in common use include:
- fumarase
- L-malate hydro-lyase
- (S)-malate hydro-lyase
Mechanism


Zwitterion
In chemistry, a zwitterion is a neutral molecule with a positive and a negative electrical charge at different locations within that molecule. Zwitterions are sometimes also called inner salts.-Examples:...
ic A-/BH+ state. E1 binds fumarate and facilitates its tansformation into malate, and E2 binds malate and facilitates its transformation into fumarate. The two forms must undergo isomerization with each catalytic turnover.
Despite its biological significance, the reaction mechanism of fumarase is not completely understood. The reaction itself can be monitored in either direction; however, it is the formation of fumarate from S-malate in particular that is less understood due to the high pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
value of the HR (Fig. 1) atom that is removed without the aid of any cofactors
Cofactor (biochemistry)
A cofactor is a non-protein chemical compound that is bound to a protein and is required for the protein's biological activity. These proteins are commonly enzymes, and cofactors can be considered "helper molecules" that assist in biochemical transformations....
or coenzymes. However, the reaction from fumarate to L-malate is better understood, and involves a stereospecific hydration of fumarate to produce S-malate by trans-addition of a hydroxyl
Hydroxyl
A hydroxyl is a chemical group containing an oxygen atom covalently bonded with a hydrogen atom. In inorganic chemistry, the hydroxyl group is known as the hydroxide ion, and scientists and reference works generally use these different terms though they refer to the same chemical structure in...
group and a hydrogen atom through a trans 1,4 addition of a hydroxyl group. Early research into this reaction suggested that the formation of fumarate from S-malate involved dehydration of malate to a carbocationic intermediate, which then loses the alpha proton to form fumarate. This led to the conclusion that in the formation of S-Malate from fumarate E1 elimination, protonation of fumarate to the carbocation was followed by the additional of a hydroxyl group from H2O. However, more recent trials have provided evidence that the mechanism actually takes place through an acid-base catalyzed elimination by means of a carbanionic intermediate E1CB elimination
E1cB elimination reaction
The E1cB elimination reaction is a special type of elimination reaction in organic chemistry. This reaction mechanism explains the formation of alkenes from alkyl halides through a carbanion intermediate given specified reaction conditions and specified substrates. The abbreviation stands for ...
(Figure 2).
Biochemical pathway
The function of fumarase in the citric acid cycleCitric acid cycle
The citric acid cycle — also known as the tricarboxylic acid cycle , the Krebs cycle, or the Szent-Györgyi-Krebs cycle — is a series of chemical reactions which is used by all aerobic living organisms to generate energy through the oxidization of acetate derived from carbohydrates, fats and...
is to facilitate a transition step in the production of energy in the form of NADH. In the cytosol
Cytosol
The cytosol or intracellular fluid is the liquid found inside cells, that is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into compartments....
the enzyme functions to metabolize fumarate, which ends up as a byproduct of the urea cycle
Urea cycle
The urea cycle is a cycle of biochemical reactions occurring in many animals that produces urea from ammonia . This cycle was the first metabolic cycle discovered , five years before the discovery of the TCA cycle...
as well as amino acid catabolism. Studies have revealed that the active site is composed of amino acid residues from three of the four subunits within the tetrameric enzyme.
The primary binding site on fumarase is known as catalytic site A. Studies have revealed that catalytic site A is composed of amino acid residues from three of the four subunits within the tetrameric enzyme. Two potential acid-base catalytic residues in the reaction include His 188 and Lys 324.
Subtypes
There are two classes of fumarases. Classifications depend on the arrangement of their relative subunit, their metal requirement, and their thermal stability. These include class I and class II. Class I fumarases are able to change state or become inactive when subjected to heat or radiation, are sensitive to superoxide anion, are Iron II (Fe2+) dependent, and are dimeric proteins consisting of around 120 kD. Class II fumarases, found in prokaryotes as well as in eukaryotes, are tetrameric enzymes of 200,000 D that contain three distinct segments of significantly homologous amino acids. They are also iron-independent and thermal-stable. Prokaryotes are known to have three different forms of fumarase: Fumarase A, Fumarase B, and Fumarase C. Fumarase C is a part of the class II fumarases, whereas Fumarase A and Fumarase B from Escherichia coli (E. coli) are classified as class I.Clinical significance
Fumarase deficiencyFumarase deficiency
Fumarase deficiency , also known as "Polygamist Down's", is an autosomal recessive metabolic disorder characterized by a deficiency of the enzyme fumarate hydratase, which is indicated by a build up of fumaric acid in the urine....
is characterized by polyhydramnios
Polyhydramnios
Polyhydramnios is a medical condition describing an excess of amniotic fluid in the amniotic sac. It is seen in 0.2 to 1.6% of pregnancies,,...
and fetal brain abnormalities. In the newborn period, findings include severe neurologic abnormalities, poor feeding, failure to thrive, and hypotonia
Hypotonia
Hypotonia is a state of low muscle tone , often involving reduced muscle strength. Hypotonia is not a specific medical disorder, but a potential manifestation of many different diseases and disorders that affect motor nerve control by the brain or muscle strength...
. Fumarase deficiency is suspected in infants with multiple severe neurologic abnormalities in the absence of an acute metabolic crisis. Inactivity of both cytosolic and mitochondrial forms of fumarase are potential causes. Isolated, increased concentration of fumaric acid
Fumaric acid
Fumaric acid or trans-butenedioic acid is the chemical compound with the formula HO2CCH=CHCO2H. This white crystalline compound is one of two isomeric unsaturated dicarboxylic acids, the other being maleic acid. In fumaric acid the carboxylic acid groups are trans and in maleic acid they are cis...
on urine organic acid analysis is highly suggestive of fumarase deficiency. Molecular genetic testing for fumarase deficiency is currently available.
Fumarase is prevalent in both fetal and adult tissues. A large percentage of the enzyme is expressed in the skin
Skin
-Dermis:The dermis is the layer of skin beneath the epidermis that consists of connective tissue and cushions the body from stress and strain. The dermis is tightly connected to the epidermis by a basement membrane. It also harbors many Mechanoreceptors that provide the sense of touch and heat...
, parathyroid, lymph
Lymph
Lymph is considered a part of the interstitial fluid, the fluid which lies in the interstices of all body tissues. Interstitial fluid becomes lymph when it enters a lymph capillary...
, and colon
Colon (anatomy)
The colon is the last part of the digestive system in most vertebrates; it extracts water and salt from solid wastes before they are eliminated from the body, and is the site in which flora-aided fermentation of unabsorbed material occurs. Unlike the small intestine, the colon does not play a...
. Mutations in the production and development of fumarase have led to the discovery of several fumarase-related diseases in humans. These include benign
Benign
A benign tumor is a tumor that lacks the ability to metastasize. Common examples of benign tumors include moles and uterine fibroids.The term "benign" implies a mild and nonprogressive disease. Indeed, many kinds of benign tumors are harmless to human health...
mesenchymal tumors of the uterus, leiomyomatosis and renal cell carcinoma
Carcinoma
Carcinoma is the medical term for the most common type of cancer occurring in humans. Put simply, a carcinoma is a cancer that begins in a tissue that lines the inner or outer surfaces of the body, and that generally arises from cells originating in the endodermal or ectodermal germ layer during...
, and fumarase deficiency
Fumarase deficiency
Fumarase deficiency , also known as "Polygamist Down's", is an autosomal recessive metabolic disorder characterized by a deficiency of the enzyme fumarate hydratase, which is indicated by a build up of fumaric acid in the urine....
. Germinal mutations in fumarase are associated with two distinct conditions. If the enzyme has missense mutation and in-frame deletions from the 3’ end, fumarase deficiency results. If it contains heterozygous 5’ missense mutation and deletions (ranging from one base pair to the whole gene), then leiomyomatosis and renal cell carcinoma/Reed’s syndrome (multiple cutaneous
Multiple cutaneous leiomyoma
Multiple cutaneous leiomyomas arise from the arrectores pilorum muscles, and are made up of a poorly circumscribed proliferation of haphazardly arranged smooth muscle fibers located in the dermis that appear to infiltrate the surrounding tissue and may extend into the subcutis.- See also :*...
and uterine leiomyomatosis) could result.
Protein
Crystal structures of fumarase C from Escherichia coliEscherichia coli
Escherichia coli is a Gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms . Most E. coli strains are harmless, but some serotypes can cause serious food poisoning in humans, and are occasionally responsible for product recalls...
have been observed to have two occupied dicarboxylate binding sites. These are known as the active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
and the B site. The active site and B site are both identified as having areas unoccupied by a bound ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
. This so-called ‘free’ crystal structure demonstrates conservation of the active-site water. Similar orientation has been discovered in other fumarase C crystal structures. Crystallographic research on the B site of the enzyme has observed that there is a shift on His129. This information suggests that water is a permanent component of the active site. It also suggests that the use of an imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
-imidazolium conversion controls access to the allosteric B site.

