
Combustion
Encyclopedia

Exothermic
In thermodynamics, the term exothermic describes a process or reaction that releases energy from the system, usually in the form of heat, but also in the form of light , electricity , or sound...
chemical reactions between a fuel
Fuel
Fuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
and an oxidant accompanied by the production of heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
and conversion of chemical species. The release of heat can result in the production of light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
in the form of either glowing or a flame
Flame
A flame is the visible , gaseous part of a fire. It is caused by a highly exothermic reaction taking place in a thin zone...
. Fuels of interest often include organic compounds (especially hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s) in the gas, liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
or solid phase.
In a complete combustion reaction, a compound reacts with an oxidizing element, such as oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
or fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
, and the products are compounds of each element in the fuel with the oxidizing element. For example:
- + 2 → + 2 H2O + energy
- CH2S + 6 → + 2 +
A simple example can be seen in the combustion of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, which is a commonly used reaction in rocket engines:
- 2 + → 2 H2O(g) + heat
The result is water vapor.
Complete combustion is almost impossible to achieve. In reality, as actual combustion reactions come to equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
, a wide variety of major and minor species will be present such as carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
and pure carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
(soot
Soot
Soot is a general term that refers to impure carbon particles resulting from the incomplete combustion of a hydrocarbon. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolyzed fuel particles such as cenospheres,...
or ash). Additionally, any combustion in atmospheric
Atmosphere
An atmosphere is a layer of gases that may surround a material body of sufficient mass, and that is held in place by the gravity of the body. An atmosphere may be retained for a longer duration, if the gravity is high and the atmosphere's temperature is low...
air, which is 78% nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
, will also create several forms of nitrogen oxides.
Complete vs. incomplete
In complete combustion, the reactant burns in oxygen, producing a limited number of products. When a hydrocarbonHydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
burns in oxygen, the reaction will only yield carbon dioxide and water. When elements are burned, the products are primarily the most common oxides. Carbon will yield carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, nitrogen will yield nitrogen dioxide
Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula it is one of several nitrogen oxides. is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent...
, sulfur will yield sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
, and iron will yield iron(III) oxide
Iron(III) oxide
Iron oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron oxide , which is rare, and iron oxide , which also occurs naturally as the mineral magnetite. As the mineral known as hematite, Fe2O3 is the main...
.
Combustion is not necessarily favorable to the maximum degree of oxidation and it can be temperature-dependent. For example, sulfur trioxide
Sulfur trioxide
Sulfur trioxide is the chemical compound with the formula SO3. In the gaseous form, this species is a significant pollutant, being the primary agent in acid rain. It is prepared on massive scales as a precursor to sulfuric acid.-Structure and bonding:Gaseous SO3 is a trigonal planar molecule of...
is not produced quantitatively in combustion of sulfur. Nitrogen oxides start to form above 2800 °F (1,537.8 °C) and more nitrogen oxides are produced at higher temperatures. Below this temperature, molecular nitrogen (N2) is favored. It is also a function of oxygen excess.
In most industrial applications and in fire
Fire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
s, air is the source of oxygen (O2). In air, each mole of oxygen is mixed with approximately 3.76 mole of nitrogen
Nitrogen
Nitrogen is a chemical element that has the symbol N, atomic number of 7 and atomic mass 14.00674 u. Elemental nitrogen is a colorless, odorless, tasteless, and mostly inert diatomic gas at standard conditions, constituting 78.08% by volume of Earth's atmosphere...
. Nitrogen does not take part in combustion, but at high temperatures, some nitrogen will be converted to NOx, usually between 1% and 0.002% (2 ppm). Furthermore, when there is any incomplete combustion, some of carbon is converted to carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. A more complete set of equations for combustion of methane in air is therefore:
- + 2 → + 2 H2O
- 2 + 3 → 2 + 4 H2O
- + → 2
- + 2 → 2
Incomplete
Incomplete combustion will only occur when there is not enough oxygen to allow the fuel to react completely to produce carbon dioxide and water. It also happens when the combustion is quenched by a heat sink such as a solid surface or flame trap.For most fuels, such as diesel oil, coal or wood, pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
occurs before combustion. In incomplete combustion, products of pyrolysis remain unburnt and contaminate the smoke with noxious particulate matter and gases. Partially oxidized compounds are also a concern; partial oxidation of ethanol can produce harmful acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
, and carbon can produce toxic carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
.
The quality of combustion can be improved by design of combustion devices, such as burner
Oil burner
An oil burner is a heating device which burns fuel oil. The oil is atomized in to a fine spray usually by forcing it under pressure through a nozzle...
s and internal combustion engines. Further improvements are achievable by catalytic after-burning devices (such as catalytic converter
Catalytic converter
A catalytic converter is a device used to convert toxic exhaust emissions from an internal combustion engine into non-toxic substances. Inside a catalytic converter, a catalyst stimulates a chemical reaction in which noxious byproducts of combustion are converted to less toxic substances by dint...
s) or by the simple partial return of the exhaust gases into the combustion process. Such devices are required by environmental legislation for cars in most countries, and may be necessary in large combustion devices, such as thermal power plants, to reach legal emission standards.
The degree of combustion can be measured and analyzed, with test equipment. HVAC
HVAC
HVAC refers to technology of indoor or automotive environmental comfort. HVAC system design is a major subdiscipline of mechanical engineering, based on the principles of thermodynamics, fluid mechanics, and heat transfer...
contractors, firemen
Firefighter
Firefighters are rescuers extensively trained primarily to put out hazardous fires that threaten civilian populations and property, to rescue people from car incidents, collapsed and burning buildings and other such situations...
and engineers use combustion analyzers to test the efficiency
Fuel efficiency
Fuel efficiency is a form of thermal efficiency, meaning the efficiency of a process that converts chemical potential energy contained in a carrier fuel into kinetic energy or work. Overall fuel efficiency may vary per device, which in turn may vary per application, and this spectrum of variance is...
of a burner during the combustion process. In addition, the efficiency of an internal combustion engine can be measured in this way, and some states and local municipalities are using combustion analysis to define and rate the efficiency of vehicles on the road today.
Smoldering
Smoldering is the slow, low-temperature, flameless form of combustion, sustained by the heat evolved when oxygen directly attacks the surface of a condensed-phase fuel. It is a typically incomplete combustion reaction. Solid materials that can sustain a smoldering reaction include coal, cellulose, wood, cotton, tobacco, peat, duff, humus, synthetic foams, charring polymers including polyurethane foam, and dust. Common examples of smoldering phenomena are the initiation of residential fires on upholstered furniture by weak heat sources (e.g., a cigarette, a short-circuited wire), and the persistent combustion of biomass behind the flaming front of wildfiresRapid
Rapid combustion is a form of combustion, otherwise known as a fireFire
Fire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
, in which large amounts of heat and light
Light
Light or visible light is electromagnetic radiation that is visible to the human eye, and is responsible for the sense of sight. Visible light has wavelength in a range from about 380 nanometres to about 740 nm, with a frequency range of about 405 THz to 790 THz...
energy are released, which often results in a flame
Flame
A flame is the visible , gaseous part of a fire. It is caused by a highly exothermic reaction taking place in a thin zone...
. This is used in a form of machinery such as internal combustion engine
Internal combustion engine
The internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
s and in thermobaric weapon
Thermobaric weapon
A thermobaric weapon, which includes the type known as a "fuel-air bomb", is an explosive weapon that produces a blast wave of a significantly longer duration than those produced by condensed explosives. This is useful in military applications where its longer duration increases the numbers of...
s. Sometimes, a large volume of gas is liberated in combustion besides the production of heat and light. The sudden evolution of large quantities of gas creates excessive pressure that produces a loud noise. Such a combustion is known as an explosion
Explosion
An explosion is a rapid increase in volume and release of energy in an extreme manner, usually with the generation of high temperatures and the release of gases. An explosion creates a shock wave. If the shock wave is a supersonic detonation, then the source of the blast is called a "high explosive"...
. Combustion need not involve oxygen; e.g., hydrogen burns in chlorine to form hydrogen chloride with the liberation of heat and light characteristic of combustion.
Turbulent
Combustion resulting in a turbulent flame is the most used for industrial application (e.g. gas turbines, gasoline engines, etc.) because the turbulence helps the mixing process between the fuel and oxidizer.Microgravity
Combustion processes behave differently in a microgravity environmentMicrogravity environment
The term micro-g environment is more or less a synonym of weightlessness and zero-G, but indicates that g-forces are not quite zero, just very small...
than in Earth-gravity conditions due to the lack of buoyancy
Buoyancy
In physics, buoyancy is a force exerted by a fluid that opposes an object's weight. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus a column of fluid, or an object submerged in the fluid, experiences greater pressure at the bottom of the...
. For example, a candle's flame takes the shape of a sphere. Microgravity combustion research contributes to understanding of spacecraft fire safety and diverse aspects of combustion physics.
Micro Combustion
Combustion processes which happen in very small volume are considered as micro combustionMicro combustion
Micro Combustion is the sequence of exothermic chemical reaction between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species at micro level. The release of heat can result in the production of light in the form of either glowing or a flame...
. Quenching distance plays a vital role in stabilizing the flame in such combustion chambers.
Chemical Equation
Generally, the chemical equationChemical equation
A chemical equation is the symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers...
for stoichiometric
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
burning of hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
in oxygen is
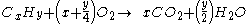
For example, the burning of propane
Propane
Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central...
is
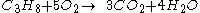
Generally, the chemical equation
Chemical equation
A chemical equation is the symbolic representation of a chemical reaction where the reactant entities are given on the left hand side and the product entities on the right hand side. The coefficients next to the symbols and formulae of entities are the absolute values of the stoichiometric numbers...
for stoichiometric
Stoichiometry
Stoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
incomplete combustion of hydrocarbon
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
in oxygen is as follows:
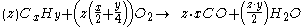
For example, the incomplete combustion of propane
Propane
Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central...
is:
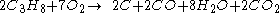
The simple word equation for the combustion of a hydrocarbon in oxygen is:
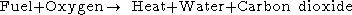
If the combustion takes place using air as the oxygen source, the nitrogen can be added to the equation,as and although it does not react, to show the composition of the flue gas:
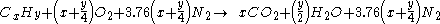
For example, the burning of propane
Propane
Propane is a three-carbon alkane with the molecular formula , normally a gas, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as a fuel for engines, oxy-gas torches, barbecues, portable stoves, and residential central...
is:

The simple word equation for this type of combustion is hydrocarbon in air:
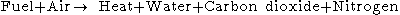
Nitrogen may also oxidize when there is an excess of oxygen. The reaction is thermodynamically favored only at high temperatures. Diesel engines are run with an excess of oxygen to combust small particles that tend to form with only a stoichiometric amount of oxygen, necessarily producing nitrogen oxide emissions. Both the United States and European Union are planning to impose limits to nitrogen oxide emissions, which necessitate the use of a special catalytic converter
Catalytic converter
A catalytic converter is a device used to convert toxic exhaust emissions from an internal combustion engine into non-toxic substances. Inside a catalytic converter, a catalyst stimulates a chemical reaction in which noxious byproducts of combustion are converted to less toxic substances by dint...
or treatment of the exhaust with urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
.
Liquid fuels
Combustion of a liquid fuel in an oxidizing atmosphere actually happens in the gas phase. It is the vapour that burns, not the liquid. Therefore, a liquid will normally catch fire only above a certain temperature: its flash pointFlash point
The flash point of a volatile material is the lowest temperature at which it can vaporize to form an ignitable mixture in air. Measuring a flash point requires an ignition source...
. The flash point of a liquid fuel is the lowest temperature at which it can form an ignitable mix with air. It is also the minimum temperature at which there is enough evaporated fuel in the air to start combustion.
Solid fuels
The act of combustion consists of three relatively distinct but overlapping phases:- Preheating phase, when the unburned fuelFuelFuel is any material that stores energy that can later be extracted to perform mechanical work in a controlled manner. Most fuels used by humans undergo combustion, a redox reaction in which a combustible substance releases energy after it ignites and reacts with the oxygen in the air...
is heated up to its flash point and then fire pointFire pointThe fire point of a fuel is the temperature at which it will continue to burn for at least 5 seconds after ignition by an open flame. At the flash point, a lower temperature, a substance will ignite briefly, but vapor might not be produced at a rate to sustain the fire...
. Flammable gases start being evolved in a process similar to dry distillationDry distillationDry distillation is the heating of solid materials to produce gaseous products . The method may or may not involve pyrolysis/thermolysis. The products are condensed and collected. This method usually requires higher temperatures than classical distillation. The method has been used to obtain...
. - Distillation phase or gaseous phase, when the mix of evolved flammable gases with oxygen is ignited. Energy is produced in the form of heat and light. FlameFlameA flame is the visible , gaseous part of a fire. It is caused by a highly exothermic reaction taking place in a thin zone...
s are often visible. Heat transfer from the combustion to the solid maintains the evolution of flammable vapours. - Charcoal phase or solid phase, when the output of flammable gases from the material is too low for persistent presence of flame and the charredCharringCharring is a chemical process of incomplete combustion of certain solids when subjected to high heat. The resulting residue matter is called Char. By the action of heat, charring removes hydrogen and oxygen from the solid, so that the remaining char is composed primarily of carbon...
fuel does not burn rapidly and just glows and later only smoulderSmoulderSmouldering is the slow, low-temperature, flameless form of combustion, sustained by the heat evolved when oxygen directly attacks the surface of a condensed-phase fuel...
s.
Reaction mechanism
Combustion in oxygen is a radicalRadical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
chain reaction where many distinct radical intermediates participate.
The high energy required for initiation is explained by the unusual structure of the dioxygen molecule. The lowest-energy configuration of the dioxygen molecule is a stable, relatively unreactive diradical in a triplet spin state
Triplet oxygen
Triplet oxygen is the ground state of the oxygen molecule. The electron configuration of the molecule has two unpaired electrons occupying two degenerate molecular orbitals...
. Bonding can be described with three bonding electron pairs and two antibonding electrons, whose spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
s are aligned, such that the molecule has nonzero total angular momentum. Most fuels, on the other hand, are in a singlet state, with paired spins and zero total angular momentum. Interaction between the two is quantum mechanically a "forbidden transition", i.e. possible with a very low probability. To initiate combustion, energy is required to force dioxygen into a spin-paired state, or singlet oxygen
Singlet oxygen
Singlet oxygen is the common name used for the diamagnetic form of molecular oxygen , which is less stable than the normal triplet oxygen. Because of its unusual properties, singlet oxygen can persist for over an hour at room temperature, depending on the environment...
. This intermediate is extremely reactive. The energy is supplied as heat
Heat
In physics and thermodynamics, heat is energy transferred from one body, region, or thermodynamic system to another due to thermal contact or thermal radiation when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between...
. The reaction produces heat, which keeps it going.
Combustion of hydrocarbons is thought to be initiated by hydrogen atom abstraction (not proton abstraction) from the fuel to oxygen, to give a hydroperoxide radical (HOO). This reacts further to give hydroperoxides, which break up to give hydroxyl radical
Hydroxyl radical
The hydroxyl radical, •OH, is the neutral form of the hydroxide ion . Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides or, in...
s. There are a great variety of these processes that produce fuel radicals and oxidizing radicals. Oxidizing species include singlet oxygen, hydroxyl, monatomic oxygen, and hydroperoxyl
Hydroperoxyl
The hydroperoxyl radical, also known as the perhydroxyl radical, is the protonated form of superoxide with the chemical formula HO2.-Reactivity:The superoxide anion, O2−, and the hydroperoxyl radical are in equilibrium in aqueous solution:...
. Such intermediates are short-lived and cannot be isolated. However, non-radical intermediates are stable and are produced in incomplete combustion. An example is acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
produced in the combustion of ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
. An intermediate in the combustion of carbon and hydrocarbons, carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
, is of special importance because it is a poisonous gas
Poison
In the context of biology, poisons are substances that can cause disturbances to organisms, usually by chemical reaction or other activity on the molecular scale, when a sufficient quantity is absorbed by an organism....
, but also economically useful for the production of syngas
Syngas
Syngas is the name given to a gas mixture that contains varying amounts of carbon monoxide and hydrogen. Examples of production methods include steam reforming of natural gas or liquid hydrocarbons to produce hydrogen, the gasification of coal, biomass, and in some types of waste-to-energy...
.
Solid fuels also undergo a great number of pyrolysis
Pyrolysis
Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
reactions that give more easily oxidized, gaseous fuels. These reactions are endothermic and require constant energy input from the combustion reactions. A lack of oxygen or other poorly designed conditions result in these noxious and carcinogenic pyrolysis products being emitted as thick, black smoke.
Temperature
Assuming perfect combustion conditions, such as complete combustion under adiabatic conditions (i.e., no heat loss or gain), the adiabatic combustion temperature can be determined. The formula that yields this temperature is based on the first law of thermodynamicsFirst law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
and takes note of the fact that the heat of combustion
Heat of combustion
The heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat...
is used entirely for heating the fuel, the combustion air or oxygen, and the combustion product gases (commonly referred to as the flue gas
Flue gas
Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases from a fireplace, oven, furnace, boiler or steam generator. Quite often, the flue gas refers to the combustion exhaust gas produced at power plants...
).
In the case of fossil fuels burnt in air, the combustion temperature depends on all of the following:
- the heating valueHeat of combustionThe heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat...
; - the stoichiometric air to fuel ratioAir-fuel ratioAir–fuel ratio is the mass ratio of air to fuel present in an internal combustion engine. If exactly enough air is provided to completely burn all of the fuel, the ratio is known as the stoichiometric mixture, often abbreviated to stoich...
 ;
; - the specific heat capacity of fuel and air;
- the air and fuel inlet temperatures.
The adiabatic combustion temperature (also known as the adiabatic flame temperature
Adiabatic flame temperature
In the study of combustion, there are two types of adiabatic flame temperature depending on how the process is completed, constant volume and constant pressure, describing the temperature the combustion products theoretically reach if no energy is lost to the outside environment.The constant volume...
) increases for higher heating values and inlet air and fuel temperatures and for stoichiometric air ratios approaching one.
Most commonly, the adiabatic combustion temperatures for coals are around 2200 °C (3,992 °F) (for inlet air and fuel at ambient temperatures and for
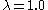 ), around 2150 °C (3,902 °F) for oil and 2000 °C (3,632 °F) for natural gas
), around 2150 °C (3,902 °F) for oil and 2000 °C (3,632 °F) for natural gasNatural gas
Natural gas is a naturally occurring gas mixture consisting primarily of methane, typically with 0–20% higher hydrocarbons . It is found associated with other hydrocarbon fuel, in coal beds, as methane clathrates, and is an important fuel source and a major feedstock for fertilizers.Most natural...
.
In industrial fired heaters
Furnace
A furnace is a device used for heating. The name derives from Latin fornax, oven.In American English and Canadian English, the term furnace on its own is generally used to describe household heating systems based on a central furnace , and sometimes as a synonym for kiln, a device used in the...
, power plant steam generators, and large gas-fired turbines
Gas turbine
A gas turbine, also called a combustion turbine, is a type of internal combustion engine. It has an upstream rotating compressor coupled to a downstream turbine, and a combustion chamber in-between....
, the more common way of expressing the usage of more than the stoichiometric combustion air is percent excess combustion air. For example, excess combustion air of 15 percent means that 15 percent more than the required stoichiometric air is being used.
Instabilities
Combustion instabilities are typically violent pressure oscillations in a combustion chamber. These pressure oscillations can be as high as 180 dB, and long term exposure to these cyclic pressure and thermal loads reduces the life of engine components. In rockets, such as the F1 used in the Saturn V program, instabilities led to massive damage of the combustion chamber and surrounding components. This problem was solved by re-designing the fuel injector. In liquid jet engines the droplet size and distribution can be used to attenuate the instabilities. Combustion instabilities are a major concern in ground-based gas turbine engines because of NOx emissions. The tendency is to run lean, an equivalence ratio less than 1, to reduce the combustion temperature and thus reduce the NOx emissions; however, running the combustion lean makes it very susceptible to combustion instabilities.The Rayleigh Criterion is the basis for analysis of thermoacoustic combustion instabilities and is evaluated using the Rayleigh Index over one cycle of instability:

where q' is the heat release rate perturbation and p' is the pressure fluctuation.
When the heat release oscillations are in phase with the pressure oscillations, the Rayleigh Index is positive and the magnitude of the thermo acoustic instability increases. On the other hand, if the Rayleigh Index is negative, then thermoacoustic damping occurs. The Rayleigh Criterion implies that a thermoacoustic instability can be optimally controlled by having heat release oscillations 180 degrees out of phase with pressure oscillations at the same frequency. This minimizes the Rayleigh Index.
Rate of Combustion
Rate of combustion is the amount of mass of a material that goes through combustion over an amount of time. It can be expressed in g/s or kg/s.See also
Related concepts- Air-fuel ratioAir-fuel ratioAir–fuel ratio is the mass ratio of air to fuel present in an internal combustion engine. If exactly enough air is provided to completely burn all of the fuel, the ratio is known as the stoichiometric mixture, often abbreviated to stoich...
- Autoignition temperatureAutoignition temperatureThe autoignition temperature or kindling point of a substance is the lowest temperature at which it will spontaneously ignite in a normal atmosphere without an external source of ignition, such as a flame or spark. This temperature is required to supply the activation energy needed for combustion...
- DeflagrationDeflagrationDeflagration is a term describing subsonic combustion that usually propagates through thermal conductivity; hot burning material heats the next layer of cold material and ignites it. Most "fire" found in daily life, from flames to explosions, is deflagration...
- DetonationDetonationDetonation involves a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations are observed in both conventional solid and liquid explosives, as well as in reactive gases...
- Dust explosionDust explosionA dust explosion is the fast combustion of dust particles suspended in the air in an enclosed location. Coal dust explosions are a frequent hazard in underground coal mines, but dust explosions can occur where any powdered combustible material is present in an enclosed atmosphere.- Conditions for...
- ExplosionExplosionAn explosion is a rapid increase in volume and release of energy in an extreme manner, usually with the generation of high temperatures and the release of gases. An explosion creates a shock wave. If the shock wave is a supersonic detonation, then the source of the blast is called a "high explosive"...
- FireFireFire is the rapid oxidation of a material in the chemical process of combustion, releasing heat, light, and various reaction products. Slower oxidative processes like rusting or digestion are not included by this definition....
- Flue gas emissions from fossil fuel combustionFlue gas emissions from fossil fuel combustionFlue-gas emissions from fossil-fuel combustion refers to the combustion product gas resulting from the much as 10 to 25 volume percent or more of the flue gas. This is closely followed in volume by water vapor created by the combustion of the hydrogen in the fuel with atmospheric oxygen...
- Flue gas stacks
- Gas stoichiometry
- Heat of combustionHeat of combustionThe heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon reacting with oxygen to form carbon dioxide, water and heat...
- Phlogiston theoryPhlogiston theoryThe phlogiston theory , first stated in 1667 by Johann Joachim Becher, is an obsolete scientific theory that postulated the existence of a fire-like element called "phlogiston", which was contained within combustible bodies and released during combustion...
(historical) - PyrolysisPyrolysisPyrolysis is a thermochemical decomposition of organic material at elevated temperatures without the participation of oxygen. It involves the simultaneous change of chemical composition and physical phase, and is irreversible...
- Pyrophoric
- Smouldering
- SootSootSoot is a general term that refers to impure carbon particles resulting from the incomplete combustion of a hydrocarbon. It is more properly restricted to the product of the gas-phase combustion process but is commonly extended to include the residual pyrolyzed fuel particles such as cenospheres,...
- Spontaneous combustionSpontaneous combustionSpontaneous combustion is the self-ignition of a mass, for example, a pile of oily rags. Allegedly, humans can also ignite and burn without an obvious cause; this phenomenon is known as spontaneous human combustion....
- StoichiometryStoichiometryStoichiometry is a branch of chemistry that deals with the relative quantities of reactants and products in chemical reactions. In a balanced chemical reaction, the relations among quantities of reactants and products typically form a ratio of whole numbers...
- Chemical looping combustionChemical looping combustionChemical looping combustion typically employs a dual fluidized bed system where a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor...
Machines and equipment
- BoilerBoilerA boiler is a closed vessel in which water or other fluid is heated. The heated or vaporized fluid exits the boiler for use in various processes or heating applications.-Materials:...
- Water heater
- Cyclone furnaceCyclone furnaceA cyclone furnace is a type of coal combustor commonly used in large industrial boilers.-Background:Developed in the early 1942 by Babcock & Wilcox to take advantage of coal grades not suitable for pulverized coal combustion, cyclone furnaces feed coal in a spiral manner into a combustion chamber...
- Bunsen burnerBunsen burnerA Bunsen burner, named after Robert Bunsen, is a common piece of laboratory equipment that produces a single open gas flame, which is used for heating, sterilization, and combustion.- Operation:...
- External combustion engineExternal combustion engineAn external combustion engine is a heat engine where an working fluid is heated by combustion in an external source, through the engine wall or a heat exchanger. The fluid then, by expanding and acting on the mechanism of the engine, produces motion and usable work...
- Industrial furnaces
- Gas turbineGas turbineA gas turbine, also called a combustion turbine, is a type of internal combustion engine. It has an upstream rotating compressor coupled to a downstream turbine, and a combustion chamber in-between....
- Internal combustion engineInternal combustion engineThe internal combustion engine is an engine in which the combustion of a fuel occurs with an oxidizer in a combustion chamber. In an internal combustion engine, the expansion of the high-temperature and high -pressure gases produced by combustion apply direct force to some component of the engine...
- Jet engineJet engineA jet engine is a reaction engine that discharges a fast moving jet to generate thrust by jet propulsion and in accordance with Newton's laws of motion. This broad definition of jet engines includes turbojets, turbofans, rockets, ramjets, pulse jets...
- Rocket engineRocket engineA rocket engine, or simply "rocket", is a jet engineRocket Propulsion Elements; 7th edition- chapter 1 that uses only propellant mass for forming its high speed propulsive jet. Rocket engines are reaction engines and obtain thrust in accordance with Newton's third law...
- Rotary combustion engine
- Staged combustion cycle (rocket)Staged combustion cycle (rocket)The staged combustion cycle, also called topping cycle or pre-burner cycle, is a thermodynamic cycle of bipropellant rocket engines. Some of the propellant is burned in a pre-burner and the resulting hot gas is used to power the engine's turbines and pumps...
Measurement techniques
- CalorimeterCalorimeterA calorimeter is a device used for calorimetry, the science of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal microcalorimeters, titration calorimeters and accelerated rate calorimeters are among the most common...
- Coherent anti-Stokes Raman spectroscopyCoherent anti-Stokes Raman spectroscopyCoherent anti-Stokes Raman spectroscopy, also called Coherent anti-Stokes Raman scattering spectroscopy , is a form of spectroscopy used primarily in chemistry, physics and related fields. It is sensitive to the same vibrational signatures of molecules as seen in Raman spectroscopy, typically the...
(CARS) - Laser Doppler velocimetryLaser Doppler velocimetryLaser Doppler Velocimetry , also known as Laser Doppler Anemometry , is the technique of using the Doppler shift in a laser beam to measure the velocity in transparent or semi-transparent fluid flows, or the linear or vibratory motion of opaque, reflecting, surfaces.-Technology origin:With the...
- Laser-induced fluorescenceLaser-induced fluorescenceLaser-induced fluorescence is a spectroscopic method used for studying structure of molecules, detection of selective species and flow visualization and measurements....
- Particle image velocimetryParticle image velocimetryParticle image velocimetry is an optical method of flow visualization used in education and research. It is used to obtain instantaneous velocity measurements and related properties in fluids...
- Thin filament pyrometryThin filament pyrometryThin Filament Pyrometry is an optical method used to measure temperatures. It involves the placement of a thin filament in a hot gas stream. Radiative emissions from the filament can be correlated with filament temperature. Filaments are typically Silicon carbide fibers with a diameter of 15...
Social applications and issues
- CookingCookingCooking is the process of preparing food by use of heat. Cooking techniques and ingredients vary widely across the world, reflecting unique environmental, economic, and cultural traditions. Cooks themselves also vary widely in skill and training...
- Cooking oilCooking oilCooking oil is purified fat of plant origin, which is usually liquid at room temperature ....
- Global warmingGlobal warmingGlobal warming refers to the rising average temperature of Earth's atmosphere and oceans and its projected continuation. In the last 100 years, Earth's average surface temperature increased by about with about two thirds of the increase occurring over just the last three decades...
External links
- Hydrocarbon combustion Simple applet that illustrates the Chemical equation.
- Simulation of gas combustion
- Theoretical and Numerical Combustion Online version of the book of T. Poinsot and D. Veynante
- Handbook of Combustion

