Heat
Encyclopedia
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
and thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, heat is energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
transferred from one body, region, or thermodynamic system
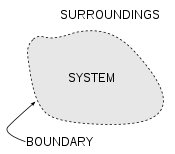
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
to another due to thermal contact
Thermal contact
In heat transfer and thermodynamics, a thermodynamic system is said to be in thermal contact with another system if it can exchange energy with it through the process of heat...
or thermal radiation
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation....
when the systems are at different temperatures. It is often described as one of the fundamental processes of energy transfer between physical entities. In this description, it is an energy transfer to a body in any other way than due to thermodynamic work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
, which is a concept of work more broad than mechanical work.
In engineering, the discipline of heat transfer
Heat transfer
Heat transfer is a discipline of thermal engineering that concerns the exchange of thermal energy from one physical system to another. Heat transfer is classified into various mechanisms, such as heat conduction, convection, thermal radiation, and phase-change transfer...
classifies energy transfer in or between systems resulting in the change of thermal energy of a system as either thermal conduction
Heat conduction
In heat transfer, conduction is a mode of transfer of energy within and between bodies of matter, due to a temperature gradient. Conduction means collisional and diffusive transfer of kinetic energy of particles of ponderable matter . Conduction takes place in all forms of ponderable matter, viz....
, first described scientifically by Joseph Fourier
Joseph Fourier
Jean Baptiste Joseph Fourier was a French mathematician and physicist best known for initiating the investigation of Fourier series and their applications to problems of heat transfer and vibrations. The Fourier transform and Fourier's Law are also named in his honour...
, by fluid convection
Convection
Convection is the movement of molecules within fluids and rheids. It cannot take place in solids, since neither bulk current flows nor significant diffusion can take place in solids....
, which is the mixing of hot and cold fluid regions due to pressure differentials, by mass transfer
Mass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
, and by thermal radiation
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation....
, the transmission of electromagnetic radiation
Electromagnetic radiation
Electromagnetic radiation is a form of energy that exhibits wave-like behavior as it travels through space...
described by black body
Black body
A black body is an idealized physical body that absorbs all incident electromagnetic radiation. Because of this perfect absorptivity at all wavelengths, a black body is also the best possible emitter of thermal radiation, which it radiates incandescently in a characteristic, continuous spectrum...
theory.
Thermodynamically, energy can only be transferred as heat between objects, or regions therein, with different temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
s, as described by the zeroth law of thermodynamics
Zeroth law of thermodynamics
The zeroth law of thermodynamics is a generalization principle of thermal equilibrium among bodies, or thermodynamic systems, in contact.The zeroth law states that if two systems are in thermal equilibrium with a third system, they are also in thermal equilibrium with each other.Systems are said to...
. This transfer happens spontaneously only in the direction to the colder body, as per the second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
. The transfer of energy by heat from one object to another object with an equal or higher temperature can happen only with the aid of a heat pump
Heat pump
A heat pump is a machine or device that effectively "moves" thermal energy from one location called the "source," which is at a lower temperature, to another location called the "sink" or "heat sink", which is at a higher temperature. An air conditioner is a particular type of heat pump, but the...
via mechanical work, or by some other similar process in which entropy is increased in the universe in a manner that compensates for the decrease of entropy in the cooled object, due to the removal of the heat from it. For example, heat may be removed against a temperature gradient by spontaneous evaporation of a liquid.
A related term is thermal energy
Thermal energy
Thermal energy is the part of the total internal energy of a thermodynamic system or sample of matter that results in the system's temperature....
, loosely defined as the energy of a body that increases with its temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
. Thermal energy is sometimes referred to as heat, although the strict definition of heat requires it to be in transfer between two systems.
Overview
Heat flows spontaneously only from systems of higher temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
to systems of lower temperature. When two systems come into thermal contact, they always exchange thermal energy due to the microscopic interactions of their particles. When the systems are at different temperatures, the net flow of thermal energy is not zero and is directed from the hotter region to the cooler region, until their temperatures are equal and the flow of heat ceases. At this point they obtain a state of thermal equilibrium
Thermal equilibrium
Thermal equilibrium is a theoretical physical concept, used especially in theoretical texts, that means that all temperatures of interest are unchanging in time and uniform in space...
, exchanging thermal energy at an equal rate in both directions.
The first law of thermodynamics
First law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
states that the energy of an isolated system
Isolated system
In the natural sciences an isolated system, as contrasted with an open system, is a physical system without any external exchange. If it has any surroundings, it does not interact with them. It obeys in particular the first of the conservation laws: its total energy - mass stays constant...
is conserved. Therefore, to change the energy of a system, energy must be transferred to or from the system. For a closed system
Closed system
-In physics:In thermodynamics, a closed system can exchange energy , but not matter, with its surroundings.In contrast, an isolated system cannot exchange any of heat, work, or matter with the surroundings, while an open system can exchange all of heat, work and matter.For a simple system, with...
, heat and work are the only mechanisms by which energy can be transferred. Work performed on a system is, by definition , an energy transfer to the system that is due to a change to external parameters of the system, such as the volume, magnetization
Magnetization
In classical electromagnetism, magnetization or magnetic polarization is the vector field that expresses the density of permanent or induced magnetic dipole moments in a magnetic material...
, center of mass in a gravitational field. Heat is the energy transferred to the system in any other way.
In the case of systems close to thermal equilibrium where notions such as the temperature can be defined, heat transfer can be related to temperature difference between systems. It is an irreversible process, which leads to the systems coming closer to mutual thermal equilibrium.
Human notions such as hot and cold are relative terms and are generally used to compare one system’s temperature to another or its surroundings.
Definitions
Scottish physicist James Clerk MaxwellJames Clerk Maxwell
James Clerk Maxwell of Glenlair was a Scottish physicist and mathematician. His most prominent achievement was formulating classical electromagnetic theory. This united all previously unrelated observations, experiments and equations of electricity, magnetism and optics into a consistent theory...
, in his 1871 classic Theory of Heat, was one of the first to enunciate a modern definition of heat. Maxwell outlined four stipulations for the definition of heat:
- It is something which may be transferred from one body to another, according to the second law of thermodynamicsSecond law of thermodynamicsThe second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
. - It is a measurable quantity, and thus treated mathematically.
- It cannot be treated as a substance, because it may be transformed into something that is not a substance, e.g., mechanical workMechanical workIn physics, work is a scalar quantity that can be described as the product of a force times the distance through which it acts, and it is called the work of the force. Only the component of a force in the direction of the movement of its point of application does work...
. - Heat is one of the forms of energyEnergyIn physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
.
Several modern definitions of heat are as follows:
- The energy transferred from a high-temperature system to a lower-temperature system is called heat.
- Any spontaneous flow of energy from one system to another caused by a difference in temperature between the systems is called heat.
In a thermodynamic sense, heat is never regarded as being stored within a system. Like work, it exists only as energy in transit from one system to another or between a system and its surroundings. When energy in the form of heat is added to a system, it is stored as kinetic and potential energy of the atoms and molecules in the system.
Notation and units
As a form of energy heat has the unit jouleJoule
The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second...
(J) in the International System of Units
International System of Units
The International System of Units is the modern form of the metric system and is generally a system of units of measurement devised around seven base units and the convenience of the number ten. The older metric system included several groups of units...
(SI). However, in many applied fields in engineering the British Thermal Unit
British thermal unit
The British thermal unit is a traditional unit of energy equal to about 1055 joules. It is approximately the amount of energy needed to heat of water, which is exactly one tenth of a UK gallon or about 0.1198 US gallons, from 39°F to 40°F...
(BTU) and the calorie
Calorie
The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule...
are often used. The standard unit for the rate of heat transferred is the watt
Watt
The watt is a derived unit of power in the International System of Units , named after the Scottish engineer James Watt . The unit, defined as one joule per second, measures the rate of energy conversion.-Definition:...
(W), defined as joules per second.
The total amount of energy transferred as heat is conventionally written as Q for algebraic purposes. Heat released by a system into its surroundings is by convention a negative quantity (Q < 0); when a system absorbs heat from its surroundings, it is positive (Q > 0). Heat transfer rate, or heat flow per unit time, is denoted by
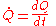 .
.Heat flux is defined as rate of heat transfer per unit cross-sectional area, resulting in the unit watts per square metre.
Semantic misconceptions
There is some debate in the scientific community regarding exactly how the term heat should be used.In current scientific usage, the language surrounding the term can be conflicting and even misleading. One study showed that several popular textbooks used language that implied several meanings of the term, that heat is the process of transferring energy, that it is the transferred energy (i.e., as if it were a substance), and that is an entity contained within a system, among other similar descriptions. The study determined it was not uncommon for a combination of these representations to appear within the same text. They found the predominant use among physicists to be as if it were a substance.
In a 2004 lecture, Friedrich Herrmann mentioned that the confusion may result from the modern practice of defining heat in terms of energy, which is at odds both with the historic scientific definitions and with the modern lay concept of heat. He argues that the quantity heat as introduced by Joseph Black
Joseph Black
Joseph Black FRSE FRCPE FPSG was a Scottish physician and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide. He was professor of Medicine at University of Glasgow . James Watt, who was appointed as philosophical instrument maker at the same university...
in the 18th century, and as used extensively by Sadi Carnot
Sadi Carnot
Sadi Carnot may refer to:*Nicolas Léonard Sadi Carnot , French physicist*Marie François Sadi Carnot , president of the third French Republic, and nephew of Nicolas Léonard Sadi Carnot...
, was in fact what is today known as entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
-- something possessed by a substance in amounts related to that substance's temperature and mass, which exits one substance and enters another in the presence of a temperature gradient and can be created in many ways but never destroyed. He further argues that the layperson's concept of heat is essentially this entropy concept, and so in redefining heat to refer to an energy concept, modern science creates an unnecessarily awkward and confusing presentation of thermal physics.
Internal energy and enthalpy
In the case where the number of particles in the system is constant, the first law of thermodynamicsFirst law of thermodynamics
The first law of thermodynamics is an expression of the principle of conservation of work.The law states that energy can be transformed, i.e. changed from one form to another, but cannot be created nor destroyed...
states that the differential change in internal energy
Internal energy
In thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
dU of a system is given by the differential heat flow δQ into the system minus the differential work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
δW exerted by the system:An alternate convention is to consider the work performed on the system by its surroundings. This leads to a change in sign of the work. This is the convention adopted by many modern textbooks of physical chemistry, such as those by Peter Atkins
Peter Atkins
Peter William Atkins is a British chemist and former Professor of Chemistry at the University of Oxford and a Fellow of Lincoln College. He is a prolific writer of popular chemistry textbooks, including Physical Chemistry, Inorganic Chemistry, and Molecular Quantum Mechanics...
and Ira Levine, but many textbooks on physics define work as work done by the system.
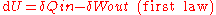 ,
,The differential transfer of heat,
 , makes differential contributions, not only to internal energy, but also to the work done by the system:
, makes differential contributions, not only to internal energy, but also to the work done by the system: ,
,The work done by the system includes boundary work, which causes the boundaries of the system to expand, in addition to other work (e.g. shaft work performed by a compressor fan):
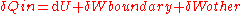 ,
,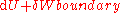 is equal to the differential enthalpy
is equal to the differential enthalpyEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
change (dH) of the system. Substitution gives:
 ,
,Both enthalpy,
 , and internal energy,
, and internal energy,  , are state function
, are state functionState function
In thermodynamics, a state function, function of state, state quantity, or state variable is a property of a system that depends only on the current state of the system, not on the way in which the system acquired that state . A state function describes the equilibrium state of a system...
s. In cyclical processes, such as the operation of a heat engine, state functions return to their initial values. Thus, the differentials for enthalpy and energy are exact differential
Exact differential
A mathematical differential is said to be exact, as contrasted with an inexact differential, if it is of the form dQ, for some differentiable function Q....
s, which are
 and
and 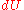 , respectively. The symbol for exact differential
, respectively. The symbol for exact differentialExact differential
A mathematical differential is said to be exact, as contrasted with an inexact differential, if it is of the form dQ, for some differentiable function Q....
s is the lowercase letter d.
In contrast, neither
 nor
nor  represents the state of the system (i.e. they need not return to their original values when returning to same step in the following cycle). Thus, the infinitesimal expressions for heat and work are inexact differentials,
represents the state of the system (i.e. they need not return to their original values when returning to same step in the following cycle). Thus, the infinitesimal expressions for heat and work are inexact differentials,  and
and  , respectively. The lowercase Greek letter delta,
, respectively. The lowercase Greek letter delta, 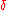 , is the symbol for inexact differential
, is the symbol for inexact differentialInexact differential
An inexact differential or imperfect differential is a specific type of differential used in thermodynamics to express the path dependence of a particular differential. It is contrasted with the concept of the exact differential in calculus, which can be expressed as the gradient of another...
s. The integral of any inexact differential over the time it takes to leave and return to the same thermodynamic state does not necessarily equal zero. However, for processes involving no change in volume (i.e.
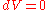 ), applied magnetic field, or other external parameters (i.e.
), applied magnetic field, or other external parameters (i.e. 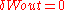 and
and  ),
),  forms the exact differential
forms the exact differentialExact differential
A mathematical differential is said to be exact, as contrasted with an inexact differential, if it is of the form dQ, for some differentiable function Q....
,
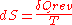 , wherein the following relation applies:
, wherein the following relation applies: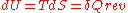 .
.Likewise, for an isentropic process
Isentropic process
In thermodynamics, an isentropic process or isoentropic process is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the...
(i.e.
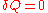 and
and 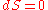 ),
),  forms the exact differential,
forms the exact differential,  , wherein the following relation applies:
, wherein the following relation applies: ,
,Path-independent examples for an ideal gas
For a simple compressible system such as an ideal gasIdeal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
inside a piston, the internal energy change
 at constant volume and the enthalpy change
at constant volume and the enthalpy change 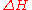 at constant pressure are modeled by separate heat capacity
at constant pressure are modeled by separate heat capacityHeat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
values, which are
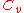 and
and 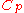 , respectively.
, respectively.Constrained to have constant volume, the heat,
 , required to change its temperature from an initial temperature, T0, to a final temperature, Tf, is given by this formula:
, required to change its temperature from an initial temperature, T0, to a final temperature, Tf, is given by this formula: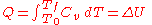
Removing the volume constraint and allowing the system to expand or contract at constant pressure, the heat,
 , required to change its temperature from an initial temperature, T0, to a final temperature, Tf, is given by this formula:
, required to change its temperature from an initial temperature, T0, to a final temperature, Tf, is given by this formula:
Note that when integrating an exact differential (e.g.
 ), the lowercase letter d is substituted for
), the lowercase letter d is substituted for  (e.g.
(e.g.  ), and when integrating an inexact differential (e.g.
), and when integrating an inexact differential (e.g.  ), the lowercase Greek letter
), the lowercase Greek letter  is removed with no replacement (e.g.
is removed with no replacement (e.g.  ).
).Incompressible substances
For incompressible substances, such as solidSolid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
s and liquid
Liquid
Liquid is one of the three classical states of matter . Like a gas, a liquid is able to flow and take the shape of a container. Some liquids resist compression, while others can be compressed. Unlike a gas, a liquid does not disperse to fill every space of a container, and maintains a fairly...
s, the distinction between the two types of heat capacity (i.e.
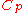 which is based on constant pressure and
which is based on constant pressure and 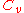 which is based on constant volume) disappears, as no work is performed.
which is based on constant volume) disappears, as no work is performed.Latent and sensible heat
In a 1847 lecture entitled On Matter, Living Force, and Heat, James Prescott JouleJames Prescott Joule
James Prescott Joule FRS was an English physicist and brewer, born in Salford, Lancashire. Joule studied the nature of heat, and discovered its relationship to mechanical work . This led to the theory of conservation of energy, which led to the development of the first law of thermodynamics. The...
characterized the terms latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
and sensible heat
Sensible heat
Sensible heat is the energy exchanged by a thermodynamic system that has as its sole effect a change of temperature.The term is used in contrast to a latent heat, which is the amount of energy exchanged that is hidden, meaning it cannot be observed as a change of temperature...
as components of heat each effecting distinct physical phenomena, namely the potential and kinetic energy of particles, respectively. He described latent energy as the energy of interaction in a given configuration of particles, i.e. a form of potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
, and the sensible heat as an energy affecting the thermal energy, which he called the living force.
Latent heat is the heat released or absorbed by a chemical substance
Chemical substance
In chemistry, a chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e. without breaking chemical bonds. They can be solids, liquids or gases.Chemical substances are...
or a thermodynamic system
Thermodynamic system
A thermodynamic system is a precisely defined macroscopic region of the universe, often called a physical system, that is studied using the principles of thermodynamics....
during a change of state
State of matter
States of matter are the distinct forms that different phases of matter take on. Solid, liquid and gas are the most common states of matter on Earth. However, much of the baryonic matter of the universe is in the form of hot plasma, both as rarefied interstellar medium and as dense...
that occurs without a change in temperature. Such a process may be a phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
, such as the melting of ice or the boiling of water. The term was introduced around 1750 by Joseph Black
Joseph Black
Joseph Black FRSE FRCPE FPSG was a Scottish physician and chemist, known for his discoveries of latent heat, specific heat, and carbon dioxide. He was professor of Medicine at University of Glasgow . James Watt, who was appointed as philosophical instrument maker at the same university...
as derived from the Latin latere (to lie hidden), characterizing its effect as not being directly measurable with a thermometer.
Sensible heat, in contrast to latent heat, is the heat exchanged by a thermodynamic system that has as its sole effect a change of temperature. Sensible heat therefore only increases the thermal energy of a system.
Specific heat
Specific heat, also called specific heat capacity, is defined as the amount of energy that has to be transferred to or from one unit of massMass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
(kilogram
Kilogram
The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water...
) or amount of substance
Amount of substance
Amount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
(mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
) to change the system temperature by one degree
Degree (temperature)
The term degree is used in several scales of temperature. The symbol ° is usually used, followed by the initial letter of the unit, for example “°C” for degree Celsius...
. Specific heat is a physical property, which means that it depends on the substance under consideration and its state as specified by its properties.
The specific heats of monatomic gases (e.g., helium) are nearly constant with temperature. Diatomic gases such as hydrogen display some temperature dependence, and triatomic gases (e.g., carbon dioxide) still more.
Entropy
In 1856, German physicist Rudolf ClausiusRudolf Clausius
Rudolf Julius Emanuel Clausius , was a German physicist and mathematician and is considered one of the central founders of the science of thermodynamics. By his restatement of Sadi Carnot's principle known as the Carnot cycle, he put the theory of heat on a truer and sounder basis...
defined the second fundamental theorem (the second law of thermodynamics
Second law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
) in the mechanical theory of heat
Theory of heat
In the history of science, the theory of heat or mechanical theory of heat was a theory, introduced predominantly in 1824 by the French physicist Sadi Carnot, that heat and mechanical work are equivalent. It is related to the mechanical equivalent of heat...
(thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
): "if two transformations which, without necessitating any other permanent change, can mutually replace one another, be called equivalent, then the generations of the quantity of heat Q from work
Work (thermodynamics)
In thermodynamics, work performed by a system is the energy transferred to another system that is measured by the external generalized mechanical constraints on the system. As such, thermodynamic work is a generalization of the concept of mechanical work in mechanics. Thermodynamic work encompasses...
at the temperature T, has the equivalence-value:"
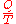
In 1865, he came to define this ratio as entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
symbolized by S, such that, for a closed, stationary system:
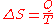
and thus, by reduction, quantities of heat δQ (an inexact differential
Inexact differential
An inexact differential or imperfect differential is a specific type of differential used in thermodynamics to express the path dependence of a particular differential. It is contrasted with the concept of the exact differential in calculus, which can be expressed as the gradient of another...
) are defined as quantities of TdS (an exact differential
Exact differential
A mathematical differential is said to be exact, as contrasted with an inexact differential, if it is of the form dQ, for some differentiable function Q....
):
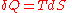
In other words, the entropy function S facilitates the quantification and measurement of heat flow through a thermodynamic boundary.
To be precise, this equality is only valid, if the heat
 is applied reversibly. If, in contrast, irreversible processes are involved, e.g. some sort of friction, then instead of the above equation one has
is applied reversibly. If, in contrast, irreversible processes are involved, e.g. some sort of friction, then instead of the above equation one has
This is the second law
Second law
Second law may refer to:* the second of Newton's laws of motion* Second law of thermodynamics* Second Law, or in Greek Deuterosis, the talmudic Mishna, which comes after the Law of Moses...
of thermodynamics.
Heat transfer in engineering

Heat transfer
Heat transfer is a discipline of thermal engineering that concerns the exchange of thermal energy from one physical system to another. Heat transfer is classified into various mechanisms, such as heat conduction, convection, thermal radiation, and phase-change transfer...
, typically considered an aspect of mechanical engineering
Mechanical engineering
Mechanical engineering is a discipline of engineering that applies the principles of physics and materials science for analysis, design, manufacturing, and maintenance of mechanical systems. It is the branch of engineering that involves the production and usage of heat and mechanical power for the...
and chemical engineering
Chemical engineering
Chemical engineering is the branch of engineering that deals with physical science , and life sciences with mathematics and economics, to the process of converting raw materials or chemicals into more useful or valuable forms...
, deals with specific applied methods by which heat transfer occurs. Note that although the definition of heat implicitly means the transfer of energy, the term
heat transfer has acquired this traditional usage in engineering and other contexts. The understanding of heat transfer is crucial for the design and operation of numerous devices and processes.
Heat transfer may occur by the mechanisms of conduction
Heat conduction
In heat transfer, conduction is a mode of transfer of energy within and between bodies of matter, due to a temperature gradient. Conduction means collisional and diffusive transfer of kinetic energy of particles of ponderable matter . Conduction takes place in all forms of ponderable matter, viz....
, radiation
Thermal radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of charged particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation....
, and mass transfer
Mass transfer
Mass transfer is the net movement of mass from one location, usually meaning a stream, phase, fraction or component, to another. Mass transfer occurs in many processes, such as absorption, evaporation, adsorption, drying, precipitation, membrane filtration, and distillation. Mass transfer is used...
. In engineering
Engineering
Engineering is the discipline, art, skill and profession of acquiring and applying scientific, mathematical, economic, social, and practical knowledge, in order to design and build structures, machines, devices, systems, materials and processes that safely realize improvements to the lives of...
, the term convective heat transfer
Convection
Convection is the movement of molecules within fluids and rheids. It cannot take place in solids, since neither bulk current flows nor significant diffusion can take place in solids....
is used to describe the combined effects of conduction and fluid flow and is often regarded as an additional mechanism of heat transfer. Although separate physical laws have been discovered to describe the behavior of each of these methods, real systems may exhibit a complicated combination. Various mathematical methods have been developed to solve or approximate the results of heat transfer in systems.
Application
In accordance with the first law, heat energy may be changed to work. This happens in so-called heat engineHeat engine
In thermodynamics, a heat engine is a system that performs the conversion of heat or thermal energy to mechanical work. It does this by bringing a working substance from a high temperature state to a lower temperature state. A heat "source" generates thermal energy that brings the working substance...
s, e.g. the steam engine
Steam engine
A steam engine is a heat engine that performs mechanical work using steam as its working fluid.Steam engines are external combustion engines, where the working fluid is separate from the combustion products. Non-combustion heat sources such as solar power, nuclear power or geothermal energy may be...
. But here the second law
Second law
Second law may refer to:* the second of Newton's laws of motion* Second law of thermodynamics* Second Law, or in Greek Deuterosis, the talmudic Mishna, which comes after the Law of Moses...
comes into play. This results in the general rule that—to keep the "lost heat" small the final temperature should be low. In contrast, so-called heat pump
Heat pump
A heat pump is a machine or device that effectively "moves" thermal energy from one location called the "source," which is at a lower temperature, to another location called the "sink" or "heat sink", which is at a higher temperature. An air conditioner is a particular type of heat pump, but the...
s can take heat at low temperatures from a "reservoir", e.g. from the soil, and deliver it by means of electrical work at a higher temperature for heating purposes. Now the temperature difference should be small, to keep the "lost electrical work" small.
See also
- Effect of sun angle on climateEffect of sun angle on climateThe amount of heat energy received at any location on the globe is a direct effect of sun angle on climate, as the angle at which sunlight strikes the Earth varies by location, time of day, and season due to the Earth's orbit around the sun and the Earth's rotation around its tilted axis...
- Heat death of the UniverseHeat death of the universeThe heat death of the universe is a suggested ultimate fate of the universe, in which the universe has diminished to a state of no thermodynamic free energy and therefore can no longer sustain motion or life. Heat death does not imply any particular absolute temperature; it only requires that...
- Heat diffusion
- Heat equationHeat equationThe heat equation is an important partial differential equation which describes the distribution of heat in a given region over time...
- Heat exchangerHeat exchangerA heat exchanger is a piece of equipment built for efficient heat transfer from one medium to another. The media may be separated by a solid wall, so that they never mix, or they may be in direct contact...
- Heat flux sensorHeat flux sensorA heat flux sensor is a transducer that generates an electrical signal proportional to the total heat rate applied to the surface of the sensor. The measured heat rate is divided by the surface area of the sensor to determine the heat flux....
- Heat transfer coefficient
- History of heatHistory of heatThe history of heat has a prominent place in the history of science. It traces its origins to the first hominids to make fire and to speculate on its operation and meaning to modern day physicists who study the microscopic nature of heat...
- Sigma heatSigma heatSigma heat, denoted S , is a is a measure of the specific energy of humid air. It is used in the field of mining engineering for calculations relating to the temperature regulation of mine air...
- Shock heating
- Thermal management of electronic devices and systemsThermal management of electronic devices and systemsHeat generated by electronic devices and circuitry must be dissipated to improve reliability and prevent premature failure. Techniques for heat dissipation can include heatsinks and fans for air cooling, and other forms of computer cooling such as liquid cooling....
- ThermometerThermometerDeveloped during the 16th and 17th centuries, a thermometer is a device that measures temperature or temperature gradient using a variety of different principles. A thermometer has two important elements: the temperature sensor Developed during the 16th and 17th centuries, a thermometer (from the...
- Relativistic heat conductionRelativistic heat conductionThe theory of relativistic heat conduction claims to be the only model for heat conduction that is compatible with the theory of special relativity, the second law of thermodynamics, electrodynamics, and quantum mechanics, simultaneously...
- Waste heatWaste heatWaste heat sometimes called Secondary heat or Low-grade heat refers to heat produced by machines, electrical equipment and industrial processes for which no useful application is found. Energy is often produced by a heat engine, running on a source of high-temperature heat...
External links
- Plasma heat at 2 gigakelvins - Article about extremely high temperature generated by scientists (Foxnews.com)
- Correlations for Convective Heat Transfer - ChE Online Resources
- An Introduction to the Quantitative Definition and Analysis of Heat written for High School Students

