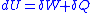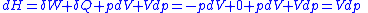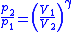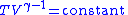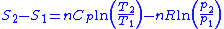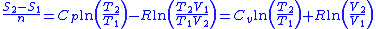Isentropic process
Encyclopedia
In thermodynamics
, an isentropic process or isoentropic process (ισον = "equal" (Greek); εντροπία entropy
= "disorder"(Greek)) is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the system remains constant. It can be proven that any reversible
adiabatic process
is an isentropic process.
states that,
where is the amount of energy the system gains by heating,
is the amount of energy the system gains by heating,  is the temperature
is the temperature
of the system, and is the change in entropy. The equal sign will hold for a reversible process
is the change in entropy. The equal sign will hold for a reversible process
. For a reversible isentropic process, there is no transfer of heat energy and therefore the process is also adiabatic
. For an irreversible process, the entropy will increase. Hence removal of heat from the system (cooling) is necessary to maintain a constant internal entropy for an irreversible process in order to make it isentropic. Thus an irreversible isentropic process is not adiabatic.
For reversible processes, an isentropic transformation is carried out by thermally "insulating" the system from its surroundings. Temperature is the thermodynamic conjugate variable
to entropy, thus the conjugate process would be an isothermal process
in which the system is thermally "connected" to a constant-temperature heat bath.
that is both adiabatic and reversible. That is, no heat is added to the flow, and no energy transformations occur due to friction
or dissipative effects
. For an isentropic flow of a perfect gas, several relations can be derived to define the pressure, density and temperature along a streamline.
Note that energy can be exchanged with the flow in an isentropic transformation, as long as it doesn't happen as heat exchange. An example of such an exchange would be an isentropic expansion or compression, with entail work done on or by the flow.
The reversible work done on a system by changing the volume is,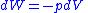
where is the pressure
is the pressure
and is the volume. The change in enthalpy
is the volume. The change in enthalpy
(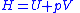 ) is given by,
) is given by,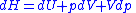
Then for a process which is both reversible and adiabatic (i.e. no heat transfer occurs),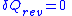 , and so
, and so 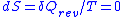 . This leads to two important observations,
. This leads to two important observations,
Next, a great deal can be computed for isentropic processes of an ideal gas. For any transformation of an ideal gas, it is always true that
Using the general results derived above for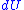 and
and  , then
, then
So for an ideal gas, the heat capacity ratio
can be written as,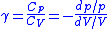
For an ideal gas is constant. Hence on integrating the above equation, assuming a perfect gas, we get
is constant. Hence on integrating the above equation, assuming a perfect gas, we get
Using the equation of state for an ideal gas,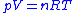 ,
,
also, for constant (per mole),
(per mole),
Thus for isentropic processes with an ideal gas,
Derived from:
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
, an isentropic process or isoentropic process (ισον = "equal" (Greek); εντροπία entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
= "disorder"(Greek)) is one in which for purposes of engineering analysis and calculation, one may assume that the process takes place from initiation to completion without an increase or decrease in the entropy of the system, i.e., the entropy of the system remains constant. It can be proven that any reversible
Reversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
adiabatic process
Adiabatic process
In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
is an isentropic process.
Background
The Second law of thermodynamicsSecond law of thermodynamics
The second law of thermodynamics is an expression of the tendency that over time, differences in temperature, pressure, and chemical potential equilibrate in an isolated physical system. From the state of thermodynamic equilibrium, the law deduced the principle of the increase of entropy and...
states that,

where
 is the amount of energy the system gains by heating,
is the amount of energy the system gains by heating,  is the temperature
is the temperatureTemperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
of the system, and
 is the change in entropy. The equal sign will hold for a reversible process
is the change in entropy. The equal sign will hold for a reversible processReversible process (thermodynamics)
In thermodynamics, a reversible process, or reversible cycle if the process is cyclic, is a process that can be "reversed" by means of infinitesimal changes in some property of the system without loss or dissipation of energy. Due to these infinitesimal changes, the system is in thermodynamic...
. For a reversible isentropic process, there is no transfer of heat energy and therefore the process is also adiabatic
Adiabatic process
In thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
. For an irreversible process, the entropy will increase. Hence removal of heat from the system (cooling) is necessary to maintain a constant internal entropy for an irreversible process in order to make it isentropic. Thus an irreversible isentropic process is not adiabatic.
For reversible processes, an isentropic transformation is carried out by thermally "insulating" the system from its surroundings. Temperature is the thermodynamic conjugate variable
Conjugate variables (thermodynamics)
In thermodynamics, the internal energy of a system is expressed in terms of pairs of conjugate variables such as temperature/entropy or pressure/volume. In fact all thermodynamic potentials are expressed in terms of conjugate pairs....
to entropy, thus the conjugate process would be an isothermal process
Isothermal process
An isothermal process is a change of a system, in which the temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir , and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir...
in which the system is thermally "connected" to a constant-temperature heat bath.
Isentropic flow
An isentropic flow is a flowFluid dynamics
In physics, fluid dynamics is a sub-discipline of fluid mechanics that deals with fluid flow—the natural science of fluids in motion. It has several subdisciplines itself, including aerodynamics and hydrodynamics...
that is both adiabatic and reversible. That is, no heat is added to the flow, and no energy transformations occur due to friction
Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and/or material elements sliding against each other. There are several types of friction:...
or dissipative effects
Dissipation
In physics, dissipation embodies the concept of a dynamical system where important mechanical models, such as waves or oscillations, lose energy over time, typically from friction or turbulence. The lost energy converts into heat, which raises the temperature of the system. Such systems are called...
. For an isentropic flow of a perfect gas, several relations can be derived to define the pressure, density and temperature along a streamline.
Note that energy can be exchanged with the flow in an isentropic transformation, as long as it doesn't happen as heat exchange. An example of such an exchange would be an isentropic expansion or compression, with entail work done on or by the flow.
Derivation of the isentropic relations
For a closed system, the total change in energy of a system is the sum of the work done and the heat added,The reversible work done on a system by changing the volume is,
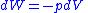
where
 is the pressure
is the pressurePressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and
 is the volume. The change in enthalpy
is the volume. The change in enthalpyEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
(
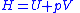 ) is given by,
) is given by,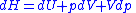
Then for a process which is both reversible and adiabatic (i.e. no heat transfer occurs),
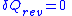 , and so
, and so 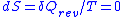 . This leads to two important observations,
. This leads to two important observations,
-
 , and
, and
Next, a great deal can be computed for isentropic processes of an ideal gas. For any transformation of an ideal gas, it is always true that
-
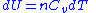 , and
, and 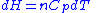 .
.
Using the general results derived above for
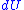 and
and  , then
, then-
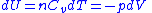 , and
, and -
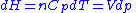 .
.
So for an ideal gas, the heat capacity ratio
Heat capacity ratio
The heat capacity ratio or adiabatic index or ratio of specific heats, is the ratio of the heat capacity at constant pressure to heat capacity at constant volume . It is sometimes also known as the isentropic expansion factor and is denoted by \gamma or \kappa . The latter symbol kappa is...
can be written as,
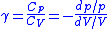
For an ideal gas
 is constant. Hence on integrating the above equation, assuming a perfect gas, we get
is constant. Hence on integrating the above equation, assuming a perfect gas, we get
-
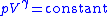 i.e.
i.e.
Using the equation of state for an ideal gas,
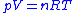 ,
,
also, for constant
 (per mole),
(per mole),
-
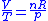 and
and 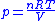
Thus for isentropic processes with an ideal gas,
-
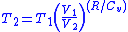 or
or 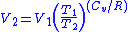
Table of isentropic relations for an ideal gas
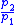 |
 |
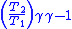 |
 |
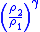 |
 |
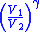 |
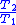 |
 |
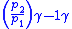 |
 |
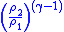 |
 |
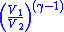 |
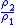 |
 |
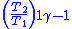 |
 |
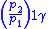 |
 |
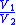 |
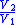 |
 |
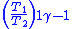 |
 |
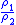 |
 |
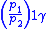 |
Derived from:
-
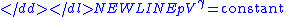
-
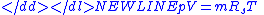
-

-
- Where:
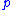 = Pressure
= Pressure = Volume
= Volume = Ratio of specific heats =
= Ratio of specific heats = 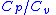
 = Temperature
= Temperature = Mass
= Mass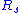 = Gas constant for the specific gas =
= Gas constant for the specific gas = 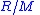
 = Universal gas constant
= Universal gas constant = Molecular weight of the specific gas
= Molecular weight of the specific gas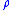 = Density
= Density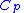 = Specific heat at constant pressure
= Specific heat at constant pressure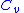 = Specific heat at constant volume
= Specific heat at constant volume
- Where:
See also
- Adiabatic processAdiabatic processIn thermodynamics, an adiabatic process or an isocaloric process is a thermodynamic process in which the net heat transfer to or from the working fluid is zero. Such a process can occur if the container of the system has thermally-insulated walls or the process happens in an extremely short time,...
- Isenthalpic processIsenthalpic processAn isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy, H; or specific enthalpy, h....
- Polytropic process
-
-
-